Effects of canagliflozin on human myocardial redox signalling: clinical implications
- PMID: 34293101
- PMCID: PMC8691807
- DOI: 10.1093/eurheartj/ehab420
Effects of canagliflozin on human myocardial redox signalling: clinical implications
Abstract
Aims: Recent clinical trials indicate that sodium-glucose cotransporter 2 (SGLT2) inhibitors improve cardiovascular outcomes in heart failure patients, but the underlying mechanisms remain unknown. We explored the direct effects of canagliflozin, an SGLT2 inhibitor with mild SGLT1 inhibitory effects, on myocardial redox signalling in humans.
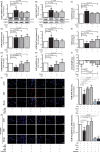
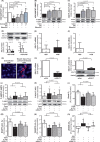
Methods and results: Study 1 included 364 patients undergoing cardiac surgery. Right atrial appendage biopsies were harvested to quantify superoxide (O2.-) sources and the expression of inflammation, fibrosis, and myocardial stretch genes. In Study 2, atrial tissue from 51 patients was used ex vivo to study the direct effects of canagliflozin on NADPH oxidase activity and nitric oxide synthase (NOS) uncoupling. Differentiated H9C2 and primary human cardiomyocytes (hCM) were used to further characterize the underlying mechanisms (Study 3). SGLT1 was abundantly expressed in human atrial tissue and hCM, contrary to SGLT2. Myocardial SGLT1 expression was positively associated with O2.- production and pro-fibrotic, pro-inflammatory, and wall stretch gene expression. Canagliflozin reduced NADPH oxidase activity via AMP kinase (AMPK)/Rac1signalling and improved NOS coupling via increased tetrahydrobiopterin bioavailability ex vivo and in vitro. These were attenuated by knocking down SGLT1 in hCM. Canagliflozin had striking ex vivo transcriptomic effects on myocardial redox signalling, suppressing apoptotic and inflammatory pathways in hCM.
Conclusions: We demonstrate for the first time that canagliflozin suppresses myocardial NADPH oxidase activity and improves NOS coupling via SGLT1/AMPK/Rac1 signalling, leading to global anti-inflammatory and anti-apoptotic effects in the human myocardium. These findings reveal a novel mechanism contributing to the beneficial cardiac effects of canagliflozin.
Keywords: AMPK; Myocardial redox state; NADPH oxidase activity; NOS coupling; SGLT1; SGLT2 inhibitor.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Figures





Comment in
-
Canagliflozin and myocardial oxidative stress: SGLT1 inhibition takes centre stage.Eur Heart J. 2021 Dec 21;42(48):4961-4963. doi: 10.1093/eurheartj/ehab519. Eur Heart J. 2021. PMID: 34370850 No abstract available.
References
-
- Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol 2012;8:495—502. - PubMed
-
- Kurosaki E, Ogasawara H. Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: preclinical and clinical data. Pharmacol Ther 2013;139:51—59. - PubMed
-
- Danne T, Biester T, Kordonouri O. Combined SGLT1 and SGLT2 inhibitors and their role in diabetes care. Diabetes Technol Ther 2018;20:S269—S277. - PubMed
-
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117—2128. - PubMed
-
- Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644—657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

