Canopy Catalysts for Alkyne Metathesis: Investigations into a Bimolecular Decomposition Pathway and the Stability of the Podand Cap
- PMID: 34293239
- PMCID: PMC8518412
- DOI: 10.1002/chem.202102080
Canopy Catalysts for Alkyne Metathesis: Investigations into a Bimolecular Decomposition Pathway and the Stability of the Podand Cap
Abstract
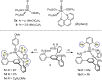
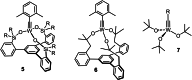
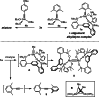
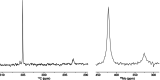
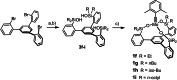
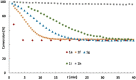
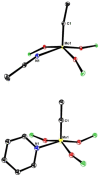
Molybdenum alkylidyne complexes with a trisilanolate podand ligand framework ("canopy catalysts") are the arguably most selective catalysts for alkyne metathesis known to date. Among them, complex 1 a endowed with a fence of lateral methyl substituents on the silicon linkers is the most reactive, although fairly high loadings are required in certain applications. It is now shown that this catalyst decomposes readily via a bimolecular pathway that engages the Mo≡CR entities in a stoichiometric triple-bond metathesis event to furnish RC≡CR and the corresponding dinuclear complex, 8, with a Mo≡Mo core. In addition to the regular analytical techniques, 95 Mo NMR was used to confirm this unusual outcome. This rapid degradation mechanism is largely avoided by increasing the size of the peripheral substituents on silicon, without unduly compromising the activity of the resulting complexes. When chemically challenged, however, canopy catalysts can open the apparently somewhat strained tripodal ligand cages; this reorganization leads to the formation of cyclo-tetrameric arrays composed of four metal alkylidyne units linked together via one silanol arm of the ligand backbone. The analogous tungsten alkylidyne complex 6, endowed with a tripodal tris-alkoxide (rather than siloxide) ligand framework, is even more susceptible to such a controlled and reversible cyclo-oligomerization. The structures of the resulting giant macrocyclic ensembles were established by single-crystal X-ray diffraction.
Keywords: alkyne metathesis; metal alkylidynes; metal-metal bonding; molybdenum; tungsten.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
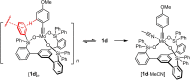





References
-
- Schrock R. R., Angew. Chem. Int. Ed. 2006, 45, 3748–3759; - PubMed
- Angew. Chem. 2006, 118, 3832–3844.
-
- Fürstner A., Davies P. W., Chem. Commun. 2005, 2307–2320. - PubMed
-
- Fürstner A., Angew. Chem. Int. Ed. 2013, 52, 2794–2819; - PubMed
- Angew. Chem. 2013, 125, 2860–2887.
-
- Fürstner A., Science 2013, 341, 1229713. - PubMed
-
- Ehrhorn H., Tamm M., Chem. Eur. J. 2019, 25, 3190–3208. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

