Management of Patients with Metastatic Castration-Sensitive Prostate Cancer in the Real-World Setting in the United States
- PMID: 34293915
- PMCID: PMC8584208
- DOI: 10.1097/JU.0000000000002121
Management of Patients with Metastatic Castration-Sensitive Prostate Cancer in the Real-World Setting in the United States
Abstract
Purpose: This study provides a contemporary assessment of the treatment patterns, health care resource utilization (HRU) and costs among metastatic castration-sensitive prostate cancer (mCSPC) patients in the U.S.
Materials and methods: Adults with mCSPC were selected from Optum's de-identified Clinformatics® Data Mart Database (Commercial insurance/Medicare Advantage [COM/MA]; January 1, 2014-July 31, 2019) or Medicare Fee-for-Service (FFS; January 1, 2014-December 31, 2017). The index date was the first metastatic disease diagnosis date on/after the first prostate cancer diagnosis (without prior evidence of castration resistance). Patients were observed for 12 months pre-index (baseline) through their mCSPC period (from index until castration resistance or followup end). First-line (1L) mCSPC therapy was assessed during the mCSPC period; all-cause HRU and health plan-paid costs per-patient-per-year (PPPY) were measured during baseline and mCSPC periods.
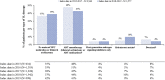
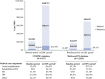
Results: Among 6,517 COM/MA and 13,324 Medicare-FFS mCSPC patients over ∼10 months (median mCSPC period), 38% and 48% remained untreated/deferred treatment, and 45% and 46% received 1L androgen deprivation therapy (ADT) monotherapy, respectively. 1L abiraterone acetate and docetaxel were used among 7% and 6% of COM/MA and 1% and 2% of Medicare-FFS patients, respectively. HRU increased from baseline to mCSPC period, resulting in increased health plan-paid costs from $21,201 to $108,767 in COM/MA and from $16,819 to $69,639 PPPY in Medicare-FFS.
Conclusions: This study highlights the limited use of newer therapies that improve survival in men with mCSPC in the U.S. HRU and costs increased substantially after onset of metastasis. Given the emergence of newer effective mCSPC therapies, further evaluation of future real-world mCSPC treatment patterns and outcomes is warranted.
Keywords: health care costs; neoplasm metastasis, castration; procedures and techniques utilization; prostatic neoplasms.
Figures
Comment in
-
Editorial Comment.J Urol. 2021 Dec;206(6):1429. doi: 10.1097/JU.0000000000002121.01. Epub 2021 Aug 30. J Urol. 2021. PMID: 34455827 No abstract available.
-
Management of Patients with Metastatic Castration-Sensitive Prostate Cancer in the Real-World Setting in the United States. Letter.J Urol. 2022 Apr;207(4):939. doi: 10.1097/JU.0000000000002456. Epub 2022 Jan 26. J Urol. 2022. PMID: 35080963 No abstract available.
References
-
- Chi KN, Agarwal N, Bjartell A, et al. : Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 2019; 381: 13. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

