hucMSC-derived exosomes attenuate colitis by regulating macrophage pyroptosis via the miR-378a-5p/NLRP3 axis
- PMID: 34294138
- PMCID: PMC8296541
- DOI: 10.1186/s13287-021-02492-6
hucMSC-derived exosomes attenuate colitis by regulating macrophage pyroptosis via the miR-378a-5p/NLRP3 axis
Abstract
Background: Human umbilical cord mesenchymal stem cell (hucMSC)-derived exosomes are recognized as novel cell-free therapeutic agents for inflammatory bowel disease (IBD), a condition caused by dysregulated intestinal mucosal immunity. In this event, macrophage pyroptosis, a process of cell death following the activation of NLRP3 (NOD-like receptor family, pyrin domain-containing 3) inflammasomes, is believed to partially account for inflammatory reactions. However, the role of macrophage pyroptosis in the process of hucMSC-derived exosomes alleviating colitis remains unknown. This study aimed at exploring the therapeutic effect and mechanism of hucMSC-derived exosomes on colitis repair.
Methods: In vivo, we used BALB/c mice to establish a dextran sulfate sodium (DSS)-induced colitis model and administrated hucMSC-derived exosomes intravenously to estimate its curative effect. Human myeloid leukemia mononuclear (THP-1) cells and mouse peritoneal macrophages (MPMs) were stimulated with lipopolysaccharides (LPS) and Nigericin to activate NLRP3 inflammasomes, which simulated an inflammation environment in vitro. A microRNA mimic was used to verify the role of miR-378a-5p/NLRP3 axis in the colitis repair.
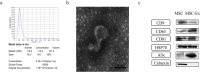
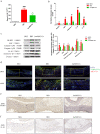
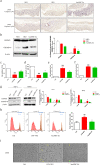
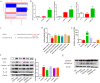
Results: hucMSC-derived exosomes inhibited the activation of NLRP3 inflammasomes in the mouse colon. The secretion of interleukin (IL)-18, IL-1β, and Caspase-1 cleavage was suppressed, resulting in reduced cell pyroptosis. The same outcome was observed in the in vitro cell experiments, where the co-culture of THP-1 cells and MPMs with hucMSC-derived exosomes caused decreased expression of NLRP3 inflammasomes and increased cell survival. Furthermore, miR-378a-5p was highly expressed in hucMSC-derived exosomes and played a vital function in colitis repair.
Conclusion: hucMSC-derived exosomes carrying miR-378a-5p inhibited NLRP3 inflammasomes and abrogated cell pyroptosis to protect against DSS-induced colitis.
Keywords: IBD; Macrophage; NLRP3; Pyroptosis; hucMSC-derived exosomes; miR-378a-5p.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

