Discrepancy of SARS-CoV-2 PCR results due to the sample collection sites and possible improper sampling
- PMID: 34294531
- PMCID: PMC8282446
- DOI: 10.1016/j.jiac.2021.07.008
Discrepancy of SARS-CoV-2 PCR results due to the sample collection sites and possible improper sampling
Abstract
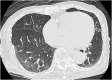
Polymerase chain reaction (PCR) testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is necessary for confirming a diagnosis of Coronavirus disease 2019 (COVID-19). Here we present a COVID-19 case of an elderly woman whose SARS-CoV-2 PCR tests showed false negative repeatedly by evaluating with different sampling sites and procedures. Nasopharyngeal swabs, suctioned sputum, and tongue swabs were collected for SARS-CoV-2-PCR. As for tongue swabs, we compared between two different sample conditions; one obtained with dry condition and the other obtained with moistened condition inside the oral cavity. SARS-CoV-2-PCR showed positive for an extended period with suctioned sputum samples compared with nasopharyngeal swabs and tongue swabs. No SARS-CoV-2 from a nasopharyngeal swab sample obtained on day 46 after symptoms onset was isolated despite high viral load (183740.5 copies/5μL). An adequate production of neutralizing antibody in a serum sample on day 46 was also confirmed. The number of RNA copies of the tongue swab samples was higher with moistened condition than with dry condition. The present case suggests that the difference of sampling site or sample condition can affect PCR results. High loads viral RNA detection does not always correlate with infectivity.
Keywords: COVID-19; False-negative; Infectivity; PCR; SARS-CoV-2.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare no conflicts of interest associated with this manuscript.
Figures


Similar articles
-
Investigation of saliva, tongue swabs and buccal swabs as alternative specimen types to nasopharyngeal swabs for SARS-CoV-2 testing.J Clin Virol. 2022 Jan;146:105053. doi: 10.1016/j.jcv.2021.105053. Epub 2021 Dec 10. J Clin Virol. 2022. PMID: 34920375 Free PMC article.
-
Value of swab types and collection time on SARS-COV-2 detection using RT-PCR assay.J Virol Methods. 2020 Dec;286:113974. doi: 10.1016/j.jviromet.2020.113974. Epub 2020 Sep 16. J Virol Methods. 2020. PMID: 32949663 Free PMC article.
-
Comparative evaluation of nasopharyngeal swab and saliva specimens for the molecular detection of SARS-CoV-2 RNA in Japanese patients with COVID-19.J Infect Chemother. 2021 Jan;27(1):126-129. doi: 10.1016/j.jiac.2020.09.027. Epub 2020 Sep 30. J Infect Chemother. 2021. PMID: 33060046 Free PMC article.
-
Detection profile of SARS-CoV-2 using RT-PCR in different types of clinical specimens: A systematic review and meta-analysis.J Med Virol. 2021 Feb;93(2):719-725. doi: 10.1002/jmv.26349. Epub 2020 Aug 2. J Med Virol. 2021. PMID: 32706393 Free PMC article.
-
Current status of the lateral flow immunoassay for the detection of SARS-CoV-2 in nasopharyngeal swabs.Biochem Med (Zagreb). 2021 Jun 15;31(2):020601. doi: 10.11613/BM.2021.020601. Biochem Med (Zagreb). 2021. PMID: 34140830 Free PMC article. Review.
Cited by
-
Symptom-based scoring technique by machine learning to predict COVID-19: a validation study.BMC Infect Dis. 2023 Dec 12;23(1):871. doi: 10.1186/s12879-023-08846-0. BMC Infect Dis. 2023. PMID: 38087249 Free PMC article.
-
Review of COVID-19 testing and diagnostic methods.Talanta. 2022 Jul 1;244:123409. doi: 10.1016/j.talanta.2022.123409. Epub 2022 Mar 31. Talanta. 2022. PMID: 35390680 Free PMC article. Review.
-
The evaluation of a rapid microfluidic immunofluorescence antigen test in detecting the infectiousness of COVID-19 patients.BMC Infect Dis. 2023 Nov 23;23(1):823. doi: 10.1186/s12879-023-08821-9. BMC Infect Dis. 2023. PMID: 37996783 Free PMC article.
-
Advances and Challenges in SARS-CoV-2 Detection: A Review of Molecular and Serological Technologies.Diagnostics (Basel). 2024 Feb 29;14(5):519. doi: 10.3390/diagnostics14050519. Diagnostics (Basel). 2024. PMID: 38472991 Free PMC article. Review.
References
-
- Ota K, Yanagihara K, Sasaki D, Kaku N, Uno N, Sakamoto K, et al. Detection of SARS-CoV-2 using qRT-PCR in saliva obtained from asymptomatic or mild COVID-19 patients, comparative analysis with matched nasopharyngeal samples. PloS One. 2021;16(6) doi: 10.1371/journal.pone.0252964. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

