Clinical and laboratory characteristics of symptomatic healthcare workers with suspected COVID-19: a prospective cohort study
- PMID: 34294751
- PMCID: PMC8298657
- DOI: 10.1038/s41598-021-93828-y
Clinical and laboratory characteristics of symptomatic healthcare workers with suspected COVID-19: a prospective cohort study
Erratum in
-
Publisher Correction: Clinical and laboratory characteristics of symptomatic healthcare workers with suspected COVID-19: a prospective cohort study.Sci Rep. 2021 Sep 23;11(1):19317. doi: 10.1038/s41598-021-99051-z. Sci Rep. 2021. PMID: 34556795 Free PMC article. No abstract available.
Abstract
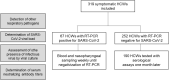
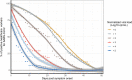
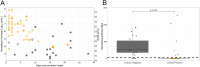
A comprehensive clinical and microbiological assessments of COVID-19 in front-line healthcare workers (HCWs) is needed. Between April 10th and May 28th, 2020, 319 HCWs with acute illness were reviewed. In addition to SARS-CoV-2 RT-PCR screening, a multiplex molecular panel was used for testing other respiratory pathogens. For SARS-CoV-2 positive HCWs, the normalized viral load, viral culture, and virus neutralization assays were performed weekly. For SARS-CoV-2 negative HCWs, SARS-CoV-2 serological testing was performed one month after inclusion. Among the 319 HCWs included, 67 (21.0%) were tested positive for SARS-CoV-2; 65/67 (97.0%) developed mild form of COVID-19. Other respiratory pathogens were found in 6/66 (9.1%) SARS-CoV-2 positive and 47/241 (19.5%) SARS-Cov-2 negative HCWs (p = 0.07). The proportion of HCWs with a viral load > 5.0 log10 cp/mL (Ct value < 25) was less than 15% at 8 days after symptom onset; 12% of HCWs were positive after 40 days (Ct > 37). More than 90% of cultivable virus had a viral load > 4.5 log10 cp/mL (Ct < 26) and were collected within 10 days after symptom onset. Among negative HCWs, 6/190 (3.2%) seroconverted. Our data suggest that the determination of viral load can be used for appreciating the infectiousness of infected HCWs. These data could be helpful for facilitating their return to work.
© 2021. The Author(s).
Conflict of interest statement
Several authors (KBP, FAF, GO, VC) are bioMérieux employees. AB has received a grant from bioMérieux and has served as consultant for bioMérieux. KBP, FAF, GO VC and AB were involved in data analysis, interpretation and wrote the article. The rest of the authors declare no conflict of interest.
Figures
References
-
- Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). https://www.who.int/publications-detail-redirect/report-of-the-who-china...).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

