Autoantibody profiles and clinical association in Thai patients with autoimmune retinopathy
- PMID: 34294798
- PMCID: PMC8298708
- DOI: 10.1038/s41598-021-94377-0
Autoantibody profiles and clinical association in Thai patients with autoimmune retinopathy
Abstract
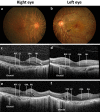
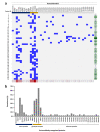
Autoimmune retinopathy (AIR) is a rare immune-mediated inflammation of the retina. The autoantibodies against retinal proteins and glycolytic enzymes were reported to be involved in the pathogenesis. This retrospective cohort study assessed the antiretinal autoantibody profiles and their association with clinical outcomes of AIR patients in Thailand. We included 44 patients, 75% were females, with the overall median age of onset of 48 (17-74, IQR 40-55.5) years. Common clinical presentations were nyctalopia (65.9%), blurred vision (52.3%), constricted visual field (43.2%), and nonrecordable electroretinography (65.9%). Underlying malignancy and autoimmune diseases were found in 2 and 12 female patients, respectively. We found 41 autoantibodies, with anti-α-enolase (65.9%) showing the highest prevalence, followed by anti-CAII (43.2%), anti-aldolase (40.9%), and anti-GAPDH (36.4%). Anti-aldolase was associated with male gender (P = 0.012, OR 7.11, 95% CI 1.54-32.91). Anti-CAII showed significant association with age of onset (P = 0.025, 95% CI - 17.28 to - 1.24), while anti-α-enolase (P = 0.002, OR 4.37, 95% CI 1.83-10.37) and anti-GAPDH (P = 0.001, OR 1.87, 95% CI 1.32-2.64) were significantly associated with nonrecordable electroretinography. Association between the antibody profiles and clinical outcomes may be used to direct and adjust the treatment plans and provide insights in the pathogenesis of AIR.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

