Cholangiocarcinoma
- PMID: 34294934
- PMCID: PMC8299326
- DOI: 10.32074/1591-951X-252
Cholangiocarcinoma
Abstract
Liver cancer represents the third leading cause of cancer-related death worldwide. Cholangiocarcinoma (CCA) is the second most common type of liver cancer after hepatocellular carcinoma, accounting for 10-15% of all primary liver malignancies. Both the incidence and mortality of CCA have been steadily increasing during the last decade. Moreover, most CCAs are diagnosed at an advanced stage, when therapeutic options are very limited.
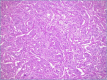
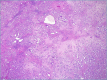
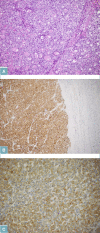
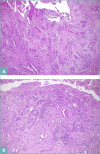
CCA may arise from any tract of the biliary system and it is classified into intrahepatic, perihilar, and distal CCA, according to the anatomical site of origin. This topographical classification also reflects distinct genetic and histological features, risk factors, and clinical outcomes. This review focuses on histopathology of CCA, its differential diagnoses, and its diagnostic pitfalls.
Keywords: biliary; cholangiocarcinoma; malignant; neoplasia; subtypes.
Copyright © 2021 Società Italiana di Anatomia Patologica e Citopatologia Diagnostica, Divisione Italiana della International Academy of Pathology.
Conflict of interest statement
The Authors declare no conflict of interest.
Figures






References
-
- Banales JM, Marin JJG, Lamarca A, et al. . Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 2020;17:557-588. https://doi.org/10.1038/s41575-020-0310-z 10.1038/s41575-020-0310-z - DOI - PMC - PubMed
-
- Cholangiocarcinoma Working Group. Italian clinical practice guidelines on cholangiocarcinoma - Part I: Classification, diagnosis and staging. Dig Liver Dis 2020;52:1282-1293. https://doi.org/10.1016/j.dld.2020.06.045 10.1016/j.dld.2020.06.045 - DOI - PubMed
-
- WHO Classification of Tumors Editorial Board. Digestive system tumors. Fifth Edition. Lyon (France): International Agency for Research on Cancer; 2019.
-
- Banales JM, Cardinale V, Carpino G, et al. . Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016;13:261-280. https://doi.org/10.1038/nrgastro.2016.51 10.1038/nrgastro.2016.51 - DOI - PubMed
-
- Rizvi S, Khan SA, Hallemeier CL, et al. . Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 2018;15:95-111. https://doi.org/10.1038/nrclinonc.2017.157 10.1038/nrclinonc.2017.157 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

