Mesenchymal tumours of the gastrointestinal tract
- PMID: 34294940
- PMCID: PMC8299319
- DOI: 10.32074/1591-951X-309
Mesenchymal tumours of the gastrointestinal tract
Abstract
Mesenchymal tumours represent a heterogenous group of neoplasms encopassing benign, intermediate malignancy, and malignant entities. Sarcomas account for approximately 1% of human malignancies. In consideration of their rarity as well as of intrinsic complexity, diagnostic accuracy represents a major challenge. Traditionally, mesenchymal tumours are regarded as lesions the occurrence of which is mostly limited to somatic soft tissues. However, the occurrence of soft tissue tumours at visceral sites represent a well recognized event, and the GI-tract ranks among the most frequently involved visceral location. There exist entities such as gastrointestinal stromal tumours (GIST) and malignant gastointestinal neuroectodermal tumors that exhibit exquisite tropism for the GI-tract. This review will focus also on other relevant clinico-pathologic entities in which occurrence at visceral location is not at all negligible.
Keywords: GIST; gastrointestinal tract; mesenchymal tumours; rare tumours; sarcoma.
Copyright © 2021 Società Italiana di Anatomia Patologica e Citopatologia Diagnostica, Divisione Italiana della International Academy of Pathology.
Conflict of interest statement
The Authors declare no conflict of interest.
Figures



















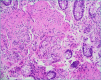
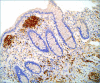
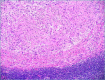




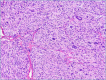




References
-
- Sbaraglia M, Dei Tos AP. The pathology of soft tissue sarcomas. Radiol Med 2019;124:266-281. https://doi.org/10.1007/s11547-018-0882-7 10.1007/s11547-018-0882-7 - DOI - PubMed
-
- Sbaraglia M, Bellan E, Dei Tos AP. The 2020 WHO Classification of Soft Tissue Tumours: news and perspectives. Pathologica 2021;113:70-84. https://doi.org/10.32074/1591-951X-213. 10.32074/1591-951X-213 Epub 2020 Nov 3. PMID: 33179614 - DOI - PMC - PubMed
-
- WHO Classification of Tumours Editorial Board. Digestive system tumours. Lyon (France): International Agency for Research on Cancer; 2019
-
- Rossi S, Gasparotto D, Toffolatti L, et al. . Molecular and clinicopathologic characterization of gastrointestinal stromal tumors (GISTs) of small size. Am J Surg Pathol 2010;34:1480-1491. https://doi.org/10.1097/PAS.0b013e3181ef7431 10.1097/PAS.0b013e3181ef7431 - DOI - PubMed
-
- Miettinen M, El-Rifai W, Sobin LHL, et al. . Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol 2002;33:478-483. https://doi.org/10.1053/hupa.2002.124123 10.1053/hupa.2002.124123 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

