Corneal Epithelial Stem Cell Supernatant in the Treatment of Severe Dry Eye Disease: A Pilot Study
- PMID: 34295148
- PMCID: PMC8291803
- DOI: 10.2147/OPTH.S322079
Corneal Epithelial Stem Cell Supernatant in the Treatment of Severe Dry Eye Disease: A Pilot Study
Abstract
Purpose: To report the subjective assessment of topical self-administered, cadaver-derived corneal epithelial stem cell supernatant for treatment of severe dry eye disease (DED).
Methods: Thirty-four eyes of 17 patients with advanced DED as defined by Standardized Patient Evaluation of Eye Dryness (SPEEDTM) questionnaire ≥14, Ocular Surface Disease Index (OSDI©) score ≥40 and documented attempt of at least six conventional dry eye therapies were enrolled into a prospective clinical trial at a single private practice institution. Treatment consisted of patient self-administered topical instillation of the corneal epithelial stem cell-derived product four times daily in both eyes for 12 weeks. Patient-reported outcome measures (PROMs) were taken with the SPEEDTM questionnaire (the main outcome variable), OSDI© score and visual analog score (VAS; UNC Dry Eye Management Scale©), and objective clinical measurements were taken with best-corrected visual acuity (BCVA), corneal topographic index measurements and tear film osmolarity. These measurements were compared at baseline versus the endpoint at completion of the 12-week treatment.
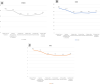
Results: All 34 eyes tolerated the treatment without any adverse events or significant side effects. Compared with baseline, both the SPEEDTM questionnaire and the VAS significantly improved at the conclusion of the 12-week treatment (p = 0.0054 and p = 0.0202, respectively). The OSDI© improved by an average of 10.9 points after the treatment but was not statistically significant (p = 0.1409). There were no significant changes in any of the objective clinical measurements. None of the study subjects failed to complete the treatment course, experienced decrease in any of the PROMs or lost one or more lines of BCVA during the follow-up period.
Conclusion: Topical corneal epithelial stem cell-derived supernatant that can be self-administered by the patient shows promise at improving patient symptoms and quality of life in the setting of severe DED that is unresponsive to conventional therapies.
Keywords: chronic ocular surface disease; conjunctival goblet cells; corneal epithelial stem cells; dry eye disease; galectin-3; glycocalyx; mucins; supernatant.
© 2021 Rush et al.
Conflict of interest statement
SWR and HD have proprietary interest in the biologic treatment studied. SWR and HD are managing partners of RegenKera, LLC which has an exclusive licensing agreement with Texas Tech University System for the patent-pending technology used to create the biologic product. SWR has a patent Stem cell supernatant manufacturing techniques pending to RegenKera, LLC. HD reports a patent WO 2020/018868 A1 pending. JC is affiliated with Oklahoma Blood Institute which has played a role in manufacturing the product used in this clinical trial. None of the investigators were paid to participate in the execution of the clinical study.
Figures
Similar articles
-
A Randomized Trial of Topical Fibrinogen-Depleted Human Platelet Lysate Treatment of Dry Eye Secondary to Chronic Graft-versus-Host Disease.Ophthalmol Sci. 2022 Jun 2;2(3):100176. doi: 10.1016/j.xops.2022.100176. eCollection 2022 Sep. Ophthalmol Sci. 2022. PMID: 36245754 Free PMC article.
-
Corneal Epithelial Thickness Correlation with Dry Eye Symptom Severity: A Cross-Sectional Study.Clin Ophthalmol. 2024 Nov 20;18:3313-3320. doi: 10.2147/OPTH.S480704. eCollection 2024. Clin Ophthalmol. 2024. PMID: 39582492 Free PMC article.
-
Mesenchymal stem cell therapy in aqueous deficient dry eye disease.Acta Ophthalmol. 2023 Oct;101 Suppl 277:3-27. doi: 10.1111/aos.15739. Acta Ophthalmol. 2023. PMID: 37840443 Review.
-
Evaluation of the Use of Highly Concentrated Autologous Platelet-Rich Plasma and Platelet-Rich Fibrin Membrane to Improve the Outcome in the Management of Severe Dry Eye Disease, Corneal Neurotrophic Ulcer and Corneal Burn.Cureus. 2024 Jan 7;16(1):e51794. doi: 10.7759/cureus.51794. eCollection 2024 Jan. Cureus. 2024. PMID: 38322082 Free PMC article.
-
Intense pulsed light (IPL) therapy for the treatment of meibomian gland dysfunction.Cochrane Database Syst Rev. 2020 Mar 18;3(3):CD013559. doi: 10.1002/14651858.CD013559. Cochrane Database Syst Rev. 2020. PMID: 32182637 Free PMC article.
Cited by
-
Establishment of human corneal epithelial organoids for ex vivo modelling dry eye disease.Cell Prolif. 2024 Nov;57(11):e13704. doi: 10.1111/cpr.13704. Epub 2024 Jul 3. Cell Prolif. 2024. PMID: 38961590 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

