Taxifolin, an Inhibitor of Sortase A, Interferes With the Adhesion of Methicillin-Resistant Staphylococcal aureus
- PMID: 34295320
- PMCID: PMC8290497
- DOI: 10.3389/fmicb.2021.686864
Taxifolin, an Inhibitor of Sortase A, Interferes With the Adhesion of Methicillin-Resistant Staphylococcal aureus
Abstract
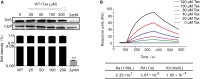
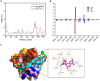
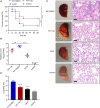
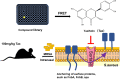
The evolution and spread of methicillin-resistant Staphylococcus aureus (MRSA) poses a significant hidden risk to human public health. The majority of antibiotics used clinically have become mostly ineffective, and so the development of novel anti-infection strategies is urgently required. Since Staphylococcus aureus (S. aureus) cysteine transpeptidase sortase A (SrtA) mediates the surface-anchoring of proteins to its surface, compounds that inhibit SrtA are considered potential antivirulence treatments. Herein, we report on the efficacy of the potent SrtA inhibitor taxifolin (Tax), a flavonoid compound isolated from Chinese herbs. It was able to reversibly block the activity of SrtA with an IC50 of 24.53 ± 0.42 μM. Tax did not display toxicity toward mammalian cells or S. aureus at a concentration of 200 μM. In addition, Tax attenuated the virulence-related phenotype of SrtA in vitro by decreasing the adherence of S. aureus, reducing the formation of a biofilm, and anchoring of S. aureus protein A on its cell wall. The mechanism of the SrtA-Tax interaction was determined using a localized surface plasmon resonance assay. Subsequent mechanistic studies confirmed that Asp-170 and Gln-172 were the principal sites on SrtA with which it binds to Tax. Importantly, in vivo experiments demonstrated that Tax protects mice against pneumonia induced by lethal doses of MRSA, significantly improving their survival rate and reducing the number of viable S. aureus in the lung tissue. The present study indicates that Tax is a useful pioneer compound for the development of novel agents against S. aureus infections.
Keywords: antivirulence; inhibitor; methicillin-resistant Staphylococcus aureus; pneumonia; sortase A; taxifolin.
Copyright © 2021 Wang, Wang, Qu, Wang, Jing, Guan, Su, Li and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Cyanidin chloride protects mice from methicillin-resistant Staphylococcus aureus-induced pneumonia by targeting Sortase A.Virulence. 2022 Dec;13(1):1434-1445. doi: 10.1080/21505594.2022.2112831. Virulence. 2022. PMID: 35983964 Free PMC article.
-
Novel inhibition of Staphylococcus aureus sortase A by plantamajoside: implications for controlling multidrug-resistant infections.Appl Environ Microbiol. 2025 Jan 31;91(1):e0180424. doi: 10.1128/aem.01804-24. Epub 2024 Dec 31. Appl Environ Microbiol. 2025. PMID: 39745463 Free PMC article.
-
Eriodictyol as a Potential Candidate Inhibitor of Sortase A Protects Mice From Methicillin-Resistant Staphylococcus aureus-Induced Pneumonia.Front Microbiol. 2021 Feb 18;12:635710. doi: 10.3389/fmicb.2021.635710. eCollection 2021. Front Microbiol. 2021. PMID: 33679670 Free PMC article.
-
Design and Synthesis of Small Molecules as Potent Staphylococcus aureus Sortase A Inhibitors.Antibiotics (Basel). 2020 Oct 16;9(10):706. doi: 10.3390/antibiotics9100706. Antibiotics (Basel). 2020. PMID: 33081148 Free PMC article. Review.
-
Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus.Mol Microbiol. 2001 Jun;40(5):1049-57. doi: 10.1046/j.1365-2958.2001.02411.x. Mol Microbiol. 2001. PMID: 11401711 Review.
Cited by
-
Chemical Characterization, Antioxidant and Antimicrobial Properties of Different Types of Tissue of Cedrus brevifolia Henry Extracts.Molecules. 2022 Apr 22;27(9):2717. doi: 10.3390/molecules27092717. Molecules. 2022. PMID: 35566066 Free PMC article.
-
Polyphenols as Inhibitors of Antibiotic Resistant Bacteria-Mechanisms Underlying Rutin Interference with Bacterial Virulence.Pharmaceuticals (Basel). 2022 Mar 21;15(3):385. doi: 10.3390/ph15030385. Pharmaceuticals (Basel). 2022. PMID: 35337182 Free PMC article.
-
Identification of novel small-molecular inhibitors of Staphylococcus aureus sortase A using hybrid virtual screening.J Antibiot (Tokyo). 2022 Jun;75(6):321-332. doi: 10.1038/s41429-022-00524-8. Epub 2022 Apr 19. J Antibiot (Tokyo). 2022. PMID: 35440771 Free PMC article.
-
Thwarting resistance: MgrA inhibition with methylophiopogonanone a unveils a new battlefront against S. aureus.NPJ Biofilms Microbiomes. 2024 Feb 27;10(1):15. doi: 10.1038/s41522-024-00485-w. NPJ Biofilms Microbiomes. 2024. PMID: 38413623 Free PMC article.
-
New Perspectives of Taxifolin in Neurodegenerative Diseases.Curr Neuropharmacol. 2023;21(10):2097-2109. doi: 10.2174/1570159X21666230203101107. Curr Neuropharmacol. 2023. PMID: 36740800 Free PMC article. Review.
References
-
- Angelis A., Hubert J., Aligiannis N., Michalea R., Abedini A., Nuzillard J.-M., et al. (2016). Bio-guided isolation of methanol-soluble metabolites of common spruce (Picea abies) bark by-products and investigation of their dermo-cosmetic properties. Molecules 21:1586. 10.3390/molecules21111586 - DOI - PMC - PubMed
-
- Carmichael N. J. E. O. T. (2014). European Centre for Ecotoxicology and Toxicology of Chemicals. Brussels: ECETOC, 547–548.
LinkOut - more resources
Full Text Sources

