A machine learning approach to integrating genetic and ecological data in tsetse flies (Glossina pallidipes) for spatially explicit vector control planning
- PMID: 34295362
- PMCID: PMC8288027
- DOI: 10.1111/eva.13237
A machine learning approach to integrating genetic and ecological data in tsetse flies (Glossina pallidipes) for spatially explicit vector control planning
Abstract
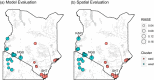
Vector control is an effective strategy for reducing vector-borne disease transmission, but requires knowledge of vector habitat use and dispersal patterns. Our goal was to improve this knowledge for the tsetse species Glossina pallidipes, a vector of human and animal African trypanosomiasis, which are diseases that pose serious health and socioeconomic burdens across sub-Saharan Africa. We used random forest regression to (i) build and integrate models of G. pallidipes habitat suitability and genetic connectivity across Kenya and northern Tanzania and (ii) provide novel vector control recommendations. Inputs for the models included field survey records from 349 trap locations, genetic data from 11 microsatellite loci from 659 flies and 29 sampling sites, and remotely sensed environmental data. The suitability and connectivity models explained approximately 80% and 67% of the variance in the occurrence and genetic data and exhibited high accuracy based on cross-validation. The bivariate map showed that suitability and connectivity vary independently across the landscape and was used to inform our vector control recommendations. Post hoc analyses show spatial variation in the correlations between the most important environmental predictors from our models and each response variable (e.g., suitability and connectivity) as well as heterogeneity in expected future climatic change of these predictors. The bivariate map suggests that vector control is most likely to be successful in the Lake Victoria Basin and supports the previous recommendation that G. pallidipes from most of eastern Kenya should be managed as a single unit. We further recommend that future monitoring efforts should focus on tracking potential changes in vector presence and dispersal around the Serengeti and the Lake Victoria Basin based on projected local climatic shifts. The strong performance of the spatial models suggests potential for our integrative methodology to be used to understand future impacts of climate change in this and other vector systems.
Keywords: disease vector; gene flow; habitat suitability; landscape genetics; random forest; spatial modeling.
© 2021 The Authors. Evolutionary Applications published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures




References
-
- Allouche, O. , Tsoar, A. , & Kadmon, R. (2006). Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). Journal of Applied Ecology, 43(6), 1223–1232. 10.1111/j.1365-2664.2006.01214.x - DOI
-
- Auguie, B. (2017). gridExtra: Miscellaneous Functions for “Grid” Graphics. Retrieved from https://cran.r‐project.org/package=gridExtra
-
- Baddeley, A. , & Turner, R. (2005). {spatstat}: An {R} package for analyzing spatial point patterns. Journal of Statistical Software, 12(6), 1–42.Retrieved from http://www.jstatsoft.org/v12/i06/
-
- Barbet‐Massin, M. , Jiguet, F. , Albert, C. H. , & Thuiller, W. (2012). Selecting pseudo‐absences for species distribution models: How, where and how many? Methods in Ecology and Evolution, 3, 327–338. 10.1111/j.2041-210X.2011.00172.x - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

