Mesenchymal Stem Cells in Treatment of Spinal Cord Injury and Amyotrophic Lateral Sclerosis
- PMID: 34295897
- PMCID: PMC8290345
- DOI: 10.3389/fcell.2021.695900
Mesenchymal Stem Cells in Treatment of Spinal Cord Injury and Amyotrophic Lateral Sclerosis
Erratum in
-
Corrigendum: Mesenchymal Stem Cells in Treatment of Spinal Cord Injury and Amyotrophic Lateral Sclerosis.Front Cell Dev Biol. 2021 Oct 28;9:770243. doi: 10.3389/fcell.2021.770243. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34778276 Free PMC article.
Abstract
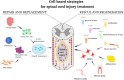
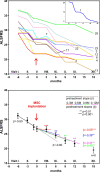
Preclinical and clinical studies with various stem cells, their secretomes, and extracellular vesicles (EVs) indicate their use as a promising strategy for the treatment of various diseases and tissue defects, including neurodegenerative diseases such as spinal cord injury (SCI) and amyotrophic lateral sclerosis (ALS). Autologous and allogenic mesenchymal stem cells (MSCs) are so far the best candidates for use in regenerative medicine. Here we review the effects of the implantation of MSCs (progenitors of mesodermal origin) in animal models of SCI and ALS and in clinical studies. MSCs possess multilineage differentiation potential and are easily expandable in vitro. These cells, obtained from bone marrow (BM), adipose tissue, Wharton jelly, or even other tissues, have immunomodulatory and paracrine potential, releasing a number of cytokines and factors which inhibit the proliferation of T cells, B cells, and natural killer cells and modify dendritic cell activity. They are hypoimmunogenic, migrate toward lesion sites, induce better regeneration, preserve perineuronal nets, and stimulate neural plasticity. There is a wide use of MSC systemic application or MSCs seeded on scaffolds and tissue bridges made from various synthetic and natural biomaterials, including human decellularized extracellular matrix (ECM) or nanofibers. The positive effects of MSC implantation have been recorded in animals with SCI lesions and ALS. Moreover, promising effects of autologous as well as allogenic MSCs for the treatment of SCI and ALS were demonstrated in recent clinical studies.
Keywords: amyotrophic lateral sclerosis; biomaterials; cell therapy; conditioned medium; exosomes; mesenchymal stem cells; neurodegenerative diseases; spinal cord injury.
Copyright © 2021 Sykova, Cizkova and Kubinova.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Ahmadian Kia N., Bahrami A. R., Ebrahimi M., Matin M. M., Neshati Z., Almohaddesin M. R., et al. (2011). Comparative analysis of chemokine receptor’s expression in mesenchymal stem cells derived from human bone marrow and adipose tissue. J. Mol. Neurosci. 44 178–185. 10.1007/s12031-010-9446-6 - DOI - PubMed
-
- Ahuja C. S., Wilson J. R., Nori S., Kotter M. R. N., Druschel C., Curt A., et al. (2017). Traumatic spinal cord injury. Nat. Rev. Dis. Primers. 3:17018. - PubMed
-
- Asadi-Golshan R., Razban V., Mirzaei E., Rahmanian A., Khajeh S., Mostafavi-Pour Z., et al. (2018). Sensory and motor behavior evidences supporting the usefulness of conditioned medium from dental pulp-derived stem cells in spinal cord injury in rats. Asian Spine J. 12 785–793. 10.31616/asj.2018.12.5.785 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

