Assessment of North American Clinical Research Site Performance During the Start-up of Large Cardiovascular Clinical Trials
- PMID: 34297072
- PMCID: PMC9435961
- DOI: 10.1001/jamanetworkopen.2021.17963
Assessment of North American Clinical Research Site Performance During the Start-up of Large Cardiovascular Clinical Trials
Abstract
Importance: Randomized clinical trials (RCTs) are critical in advancing patient care, yet conducting such large-scale trials requires tremendous resources and coordination. Clinical site start-up performance metrics can provide insight into opportunities for improved trial efficiency but have not been well described.
Objective: To measure the start-up time needed to reach prespecified milestones across sites in large cardiovascular RCTs in North America and to evaluate how these metrics vary by time and type of regulatory review process.
Design, setting, and participants: This cohort study evaluated cardiovascular RCTs conducted from July 13, 2004, to February 1, 2017. The RCTs were coordinated by a single academic research organization, the Duke Clinical Research Institute. Nine consecutive trials with completed enrollment and publication of results in their target journal were studied. Data were analyzed from December 4, 2019, to January 11, 2021.
Exposures: Year of trial enrollment initiation (2004-2007 vs 2008-2012) and use of a central vs local institutional review board (IRB).
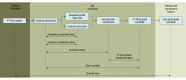
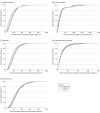
Main outcomes and measures: The primary outcome was the median start-up time (from study protocol delivery to first participant enrollment) as compared by trial year and type of IRB used. The median start-up time for the top 10% of sites was also reported. Secondary outcomes included time to site regulatory approval, time to contract execution, and time to site activation.
Results: For the 9 RCTs included, the median site start-up time shortened only slightly over time from 267 days (interquartile range [IQR], 185-358 days) for 2004-2007 trials to 237 days (IQR, 162-343 days) for 2008-2012 trials (overall median, 255 days [IQR, 177-350 days]; P < .001). For the top 10% of sites, median start-up time was 107 days (IQR, 95-121 days) for 2004-2007 trials vs 104 days (IQR, 84-118 days) for 2008-2012 trials (overall median, 106 days [IQR, 90-120 days]; P = .04). The median start-up time was shorter among sites using a central IRB (199 days [IQR, 140-292 days]) than those using a local IRB (287 days [IQR, 205-390 days]; P < .001).
Conclusions and relevance: This cohort study of North American research sites in large cardiovascular RCTs found a duration of nearly 9 months from the time of study protocol delivery to the first participant enrollment; this metric was only slightly shortened during the study period but was reduced to less than 4 months for top-performing sites. These findings suggest that the use of central IRBs has the potential to improve RCT efficiency.
Conflict of interest statement
Figures
Comment in
-
Reconfiguring the Cardiovascular Clinical Trial Enterprise in the United States.JAMA Netw Open. 2021 Jul 1;4(7):e2118176. doi: 10.1001/jamanetworkopen.2021.18176. JAMA Netw Open. 2021. PMID: 34297078 No abstract available.

