Selective blood-brain barrier permeabilization of brain metastases by a type 1 receptor-selective tumor necrosis factor mutein
- PMID: 34297105
- PMCID: PMC8730757
- DOI: 10.1093/neuonc/noab177
Selective blood-brain barrier permeabilization of brain metastases by a type 1 receptor-selective tumor necrosis factor mutein
Abstract
Background: Metastasis to the brain is a major challenge with poor prognosis. The blood-brain barrier (BBB) is a significant impediment to effective treatment, being intact during the early stages of tumor development and heterogeneously permeable at later stages. Intravenous injection of tumor necrosis factor (TNF) selectively induces BBB permeabilization at sites of brain micrometastasis, in a TNF type 1 receptor (TNFR1)-dependent manner. Here, to enable clinical translation, we have developed a TNFR1-selective agonist variant of human TNF that induces BBB permeabilization, while minimizing potential toxicity.
Methods: A library of human TNF muteins (mutTNF) was generated and assessed for binding specificity to mouse and human TNFR1/2, endothelial permeabilizing activity in vitro, potential immunogenicity, and circulatory half-life. The permeabilizing ability of the most promising variant was assessed in vivo in a model of brain metastasis.
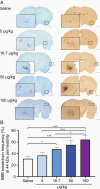
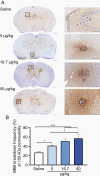
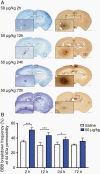
Results: The primary mutTNF variant showed similar affinity for human TNFR1 than wild-type human TNF, similar affinity for mouse TNFR1 as wild-type mouse TNF, undetectable binding to human/mouse TNFR2, low potential immunogenicity, and permeabilization of an endothelial monolayer. Circulatory half-life was similar to mouse/human TNF and BBB permeabilization was induced selectively at sites of micrometastases in vivo, with a time window of ≥24 hours and enabling delivery of agents within a therapeutically relevant range (0.5-150 kDa), including the clinically approved therapy, trastuzumab.
Conclusions: We have developed a clinically translatable mutTNF that selectively opens the BBB at micrometastatic sites, while leaving the rest of the cerebrovasculature intact. This approach will open a window for brain metastasis treatment that currently does not exist.
Keywords: blood-brain barrier; brain metastasis; mutein; permeabilization; tumor necrosis factor.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Figures