Economic Model to Evaluate the Cost-Effectiveness of Second-Line Nilotinib Versus Dasatinib for the Treatment of Philadelphia Chromosome-Positive Chronic Myeloid Leukemia (CML-CP) in Italy
- PMID: 34297312
- PMCID: PMC8807738
- DOI: 10.1007/s41669-021-00286-3
Economic Model to Evaluate the Cost-Effectiveness of Second-Line Nilotinib Versus Dasatinib for the Treatment of Philadelphia Chromosome-Positive Chronic Myeloid Leukemia (CML-CP) in Italy
Abstract
Objective: The aim of this study was to evaluate the cost effectiveness of second-line nilotinib versus dasatinib for the treatment of Philadelphia chromosome-positive chronic myeloid leukemia (CML-CP) patients who are intolerant or resistant to imatinib and can transition to treatment-free remission (TFR).
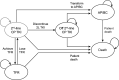
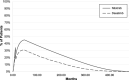
Methods: A partitioned survival model was developed to compare the cost effectiveness of nilotinib versus dasatinib. The model was developed from the Italian healthcare payer perspective and included the following health states: on second-line tyrosine kinase inhibitor (TKI), off second-line TKI, accelerated phase/blastic crisis, TFR, and death. Progression-free and overall survival curves were derived from patient-level data that compared nilotinib and dasatinib as second-line therapy in CML-CP patients who were resistant or intolerant to imatinib. Drug costs, healthcare costs, and adverse event costs were based on real-world evidence and publicly available databases. Cost effectiveness was estimated over a 40-year time horizon. Scenario analyses were performed by adjusting time horizon, TFR parameters, costs, and utilities.
Results: Second-line nilotinib resulted in greater time spent in TFR (0.91 life-years), increased quality-adjusted life-years (QALYs) (1.89), increased life-years (2.16), and decreased per-patient costs (- 38,760 €). Therefore, nilotinib was strongly dominant compared with dasatinib in the base-case analysis. Nilotinib remained strongly dominant in most scenario analyses including shorter time horizon, exclusion of TFR, and varying TKI drug costs.
Conclusions: While the model showed that nilotinib treatment of imatinib-intolerant or resistant CML-CP patients was more effective and less costly than dasatinib treatment, there is considerable uncertainty in the findings.
© 2021. The Author(s).
Conflict of interest statement
The following authors are employed by the sponsor: Vikalp Maheshwari, Gianluca Agostoni, Kalitsa Filioussi, and Ricardo Viana. Massimiliano Bonifacio receives personal fees and non-financial support from the sponsor outside the submitted work. Diana Tran is an employee of EVERSANA, which received funding from Novartis Pharmaceuticals to develop the model described in this manuscript.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

