Fast Ion-Chelate Dissociation Rate for In Vivo MRI of Labile Zinc with Frequency-Specific Encodability
- PMID: 34297566
- PMCID: PMC8397314
- DOI: 10.1021/jacs.1c05376
Fast Ion-Chelate Dissociation Rate for In Vivo MRI of Labile Zinc with Frequency-Specific Encodability
Abstract
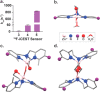
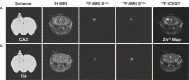
Fast ion-chelate dissociation rates and weak ion-chelate affinities are desired kinetic and thermodynamic features for imaging probes to allow reversible binding and to prevent deviation from basal ionic levels. Nevertheless, such properties often result in poor readouts upon ion binding, frequently result in low ion specificity, and do not allow the detection of a wide range of concentrations. Herein, we show the design, synthesis, characterization, and implementation of a Zn2+-probe developed for MRI that possesses reversible Zn2+-binding properties with a rapid dissociation rate (koff = 845 ± 35 s-1) for the detection of a wide range of biologically relevant concentrations. Benefiting from the implementation of chemical exchange saturation transfer (CEST), which is here applied in the 19F-MRI framework in an approach termed ion CEST (iCEST), we demonstrate the ability to map labile Zn2+ with spectrally resolved specificity and with no interference from competitive cations. Relying on fast koff rates for enhanced signal amplification, the use of iCEST allowed the designed fluorinated chelate to experience weak Zn2+-binding affinity (Kd at the mM range), but without compromising high cationic specificity, which is demonstrated here for mapping the distribution of labile Zn2+ in the hippocampal tissue of a live mouse. This strategy for accelerating ion-chelate koff rates for the enhancement of MRI signal amplifications without affecting ion specificity could open new avenues for the design of additional probes for other metal ions beyond zinc.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Que E. L.; Bleher R.; Duncan F. E.; Kong B. Y.; Gleber S. C.; Vogt S.; Chen S.; Garwin S. A.; Bayer A. R.; Dravid V. P.; Woodruff T. K.; O’Halloran T. V. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat. Chem. 2015, 7 (2), 130–9. 10.1038/nchem.2133. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

