RNA modulates physiological and neuropathological protein phase transitions
- PMID: 34297914
- PMCID: PMC8434763
- DOI: 10.1016/j.neuron.2021.06.023
RNA modulates physiological and neuropathological protein phase transitions
Abstract
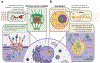
Aggregation of RNA-binding proteins (RBPs) is a pathological hallmark of neurodegenerative disorders like amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). In these diseases, TDP-43 and FUS RBPs are depleted from the nuclear compartment, where they are normally localized, and found within cytoplasmic inclusions in degenerating regions of affected individuals' postmortem tissue. The mechanisms responsible for aggregation of these proteins has remained elusive, but recent studies suggest liquid-liquid phase separation (LLPS) might serve as a critical nucleation step in formation of pathological inclusions. The process of phase separation also underlies the formation and maintenance of several functional membraneless organelles (MLOs) throughout the cell, some of which contain TDP-43, FUS, and other disease-linked RBPs. One common ligand of disease-linked RBPs, RNA, is a major component of MLOs containing RBPs and has been demonstrated to be a strong modulator of RBP phase transitions. Although early evidence suggested a largely synergistic effect of RNA on RBP phase separation and MLO assembly, recent work indicates that RNA can also antagonize RBP phase behavior under certain physiological and pathological conditions. In this review, we describe the mechanisms underlying RNA-mediated phase transitions of RBPs and examine the molecular properties of these interactions, such as RNA length, sequence, and secondary structure, that mediate physiological or pathological LLPS.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
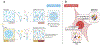

References
Numbers correspond to references listed below:
-
- Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, et al.TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008May;40(5):572–574. - PubMed
-
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al.Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006October6;314(5796):130–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

