Developing human pluripotent stem cell-based cerebral organoids with a controllable microglia ratio for modeling brain development and pathology
- PMID: 34297942
- PMCID: PMC8365109
- DOI: 10.1016/j.stemcr.2021.06.011
Developing human pluripotent stem cell-based cerebral organoids with a controllable microglia ratio for modeling brain development and pathology
Abstract
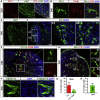
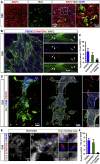
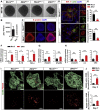
Microglia play critical roles in brain development, homeostasis, and disease. Microglia in animal models cannot accurately model human microglia due to notable transcriptomic and functional differences between human and other animal microglia. Incorporating human pluripotent stem cell (hPSC)-derived microglia into brain organoids provides unprecedented opportunities to study human microglia. However, an optimized method that integrates appropriate amounts of microglia into brain organoids at a proper time point, resembling in vivo brain development, is still lacking. Here, we report a new brain region-specific, microglia-containing organoid model by co-culturing hPSC-derived primitive neural progenitor cells and primitive macrophage progenitors. In the organoids, the number of human microglia can be controlled, and microglia exhibit phagocytic activity and synaptic pruning function. Furthermore, human microglia respond to Zika virus infection of the organoids. Our findings establish a new microglia-containing brain organoid model that will serve to study human microglial function in a variety of neurological disorders.
Keywords: Zika virus; cerebral organoid; human iPSC; human pluripotent stem cell; microglia; synaptic pruning.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

