Effectiveness of typhoid conjugate vaccine against culture-confirmed Salmonella enterica serotype Typhi in an extensively drug-resistant outbreak setting of Hyderabad, Pakistan: a cohort study
- PMID: 34297962
- PMCID: PMC8315145
- DOI: 10.1016/S2214-109X(21)00255-2
Effectiveness of typhoid conjugate vaccine against culture-confirmed Salmonella enterica serotype Typhi in an extensively drug-resistant outbreak setting of Hyderabad, Pakistan: a cohort study
Abstract
Background: Salmonella enterica serotype Typhi (S Typhi) is a major public health problem in low-income and middle-income countries. We aimed to investigate the effectiveness and impact of the typhoid conjugate vaccine Typbar-TCV against S Typhi among children in an outbreak setting of extensively drug-resistant (XDR) S Typhi in Pakistan.
Methods: This cohort study was done from Feb 21, 2018, to Dec 31, 2019. A census survey of all households located in the Qasimabad and Latifabad subdistricts of Hyderabad, Pakistan, was done at baseline, and 174 005 households were registered in the census. The Typbar-TCV immunisation campaign was initiated at temporary vaccination centres and 207 000 children aged 6 months to 10 years were vaccinated from Feb 21, 2018, to Dec 31, 2018. Social mobilisers informed parents about the vaccination process. Vaccination records were maintained electronically and linked with the household census surveys. Active surveillance for suspected and blood-culture-confirmed S Typhi was established in hospitals, clinics, and laboratories to assess the following outcomes: cases of suspected typhoid fever, culture-confirmed S Typhi, and antimicrobial resistance. An age-stratified cohort of 1100 vaccinated children was randomly selected from the vaccination registry, tested for Vi-IgG antibodies (data not reported), and followed up fortnightly (via telephone calls or household visits) until Dec 31, 2019, for ascertainment of outcomes during the study period. 20 847 vaccinated and unvaccinated children were randomly selected from the census registry as a quality control cohort and followed up from Oct 1 to Dec 31, 2019, for ascertainment of outcomes. Vaccine effectiveness against suspected, culture-confirmed, and XDR S Typhi was calculated.
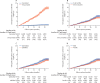
Findings: 23 407 children from the census registry and surveillance system were included in the vaccine effectiveness analysis. 13 436 (57·4%) children were vaccinated, 12 214 (52·2%) were male, and 10 168 (43·4%) were aged 6-59 months. 5378 (23·0%) of 23 407 children had suspected S Typhi, among whom 775 (14·4%) had culture-confirmed S Typhi and 361 (68·6%) of 526 had XDR S Typhi. Vaccine effectiveness was 55% (95% CI 52-57) against suspected S Typhi (regardless of culture confirmation), 95% (93-96) against culture-confirmed S Typhi, and 97% (95-98) against XDR S Typhi.
Interpretation: Typbar-TCV is effective in protecting children against S Typhi infection in an outbreak setting, and was able, with moderate deployment, to curtail a major XDR S Typhi outbreak in a densely populated setting. The vaccine shows efficacy against S Typhi irrespective of antimicrobial resistance.
Funding: Bill & Melinda Gates Foundation.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests NB-Z received investigator-initiated research grants unrelated to this work from Merck, the Serum Institute of India, and PATH Seattle. NB-Z served on the data safety monitoring board for a study at the University of Oxford, Oxford, UK (Pancreatic Enzymes and Bile Acids: A Non-Antibiotic Approach to Intestinal Dysbiosis in Acutely Ill Severely Malnourished Children. A Placebo Controlled Double Blind Randomised Controlled Trial in Kenya and Malawi), and received a nominal fee as a scientific advisory committee member for the COVID-19 Vaccines International Pregnancy Exposure Registry. NB-Z reports two discussions with Bharat Biotech International relating to the design and evaluation of a specific product. This advice was provided pro bono, and under no contractual arrangement other than non-disclosure about the product in question. These discussions are unrelated to the company's typhoid conjugate vaccine that was evaluated in this Article. All other authors declare no competing interests.
Figures

Comment in
-
Use of the typhoid conjugate vaccine in endemic settings.Lancet Glob Health. 2021 Aug;9(8):e1047-e1048. doi: 10.1016/S2214-109X(21)00308-9. Lancet Glob Health. 2021. PMID: 34297950 No abstract available.
References
-
- Sur D, Ochiai RL, Bhattacharya SK. A cluster-randomized effectiveness trial of Vi typhoid vaccine in India. N Engl J Med. 2009;361:335–344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

