REDCap on FHIR: Clinical Data Interoperability Services
- PMID: 34298155
- PMCID: PMC9217161
- DOI: 10.1016/j.jbi.2021.103871
REDCap on FHIR: Clinical Data Interoperability Services
Abstract
Background: Despite widespread use of electronic data capture (EDC) systems for research and electronic health records (EHR), most transfer of data between EHR and EDC systems is manual and error prone. Increased adoption of Health Level Seven Fast Healthcare Interoperability Resource (FHIR) application programming interfaces (APIs) in recent years by EHR systems has increased the availability of patient data for external applications such as REDCap.
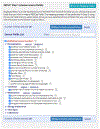
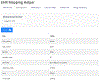
Objective: Describe the development of the REDCap Clinical Data Interoperability Services (CDIS) module that provides seamless data exchange between the REDCap research EDC and any EHR system with a FHIR API. CDIS enables end users to independently set up their data collection projects, map EHR data to fields, and adjudicate data transfer without project-by-project involvement from Health Information Technology staff.
Methods: We identified two use cases for EHR data transfer into REDCap. Clinical Data Pull (CDP) automatically pulls EHR data into user-defined REDCap fields and replaces the workflow of having to transcribe or copy and paste data from the EHR. Clinical Data Mart (CDM) collects all specified data for a patient over a given time period and replaces the process of importing EHR data for registries from research databases. With an iterative process, we designed our access control, authentication, variable selection, and mapping interfaces in such a way that end users could easily set up and use CDIS.
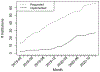
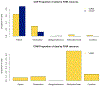
Results: Since its release, the REDCap CDIS has been used to pull over 19.5 million data points for 82 projects at Vanderbilt University Medical Center. Software and documentation are available through the REDCap Consortium.
Conclusions: The new REDCap Clinical Data and Interoperability Services (CDIS) module leverages the FHIR standard to enable real-time and direct data extraction from the EHR. Researchers can self-service the mapping and adjudication of EHR data into REDCap. The uptake of CDIS at VUMC and other REDCap consortium sites is improving the accuracy and efficiency of EHR data collection by reducing the need for manual transcription and flat file uploads.
Keywords: Clinical research; Electronic data capture; Electronic health record; Fast Healthcare Interoperability Resources.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures








References
-
- Eisenstein EL, Collins R, Cracknell BS, Podesta O, Reid ED, Sandercock P, Shakhov Y, Terrin ML, Sellers MA, Califf RM, Granger CB, Diaz R, Sensible approaches for reducing clinical trial costs, Clin. Trials 5 (2008) 75–84. - PubMed
-
- Welker JA, Implementation of electronic data capture systems: barriers and solutions, Contemp. Clin. Trials 28 (2007) 329–336. - PubMed
-
- Adler-Milstein J, Jha AK, HITECH Act Drove Large Gains In Hospital Electronic Health Record Adoption, Health Aff. . 36 (2017) 1416–1422. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

