Generation and Characterization of a New Preclinical Mouse Model of EGFR-Driven Lung Cancer with MET-Induced Osimertinib Resistance
- PMID: 34298655
- PMCID: PMC8307933
- DOI: 10.3390/cancers13143441
Generation and Characterization of a New Preclinical Mouse Model of EGFR-Driven Lung Cancer with MET-Induced Osimertinib Resistance
Abstract
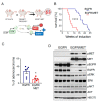
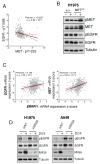
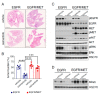
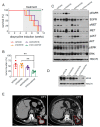
Despite the introduction of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) to treat advanced lung cancer harboring EGFR-activating mutations, the prognosis remains unfavorable because of intrinsic and/or acquired resistance. We generated a new state-of-the-art mouse strain harboring the human EGFRT790M/L858R oncogene and MET overexpression (EGFR/MET strain) that mimics the MET amplification occurring in one out of five patients with EGFR-mutated lung cancer that relapsed after treatment with osimertinib, a third-generation anti-EGFR TKI. We found that survival was reduced in EGFR/MET mice compared with mice harboring only EGFRT790M/L858R (EGFR strain). Moreover, EGFR/MET-driven lung tumors were resistant to osimertinib, recapitulating the phenotype observed in patients. Conversely, as also observed in patients, the crizotinib (anti-MET TKI) and osimertinib combination improved survival and reduced tumor burden in EGFR/MET mice, further validating the model's value for preclinical studies. We also found that in EGFR/MET mice, MET overexpression negatively regulated EGFR activity through MIG6 induction, a compensatory mechanism that allows the coexistence of the two onco-genic events. Our data suggest that single EGFR or MET inhibition might not be a good therapeutic option for EGFR-mutated lung cancer with MET amplification, and that inhibition of both pathways should be the best clinical choice in these patients.
Keywords: EGFR; MET; TKI; lung cancer; preclinical mouse models.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Sequist L.V., Han J.Y., Ahn M.J., Cho B.C., Yu H., Kim S.W., Yang J.C., Lee J.S., Su W.C., Kowalski D., et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: Interim results from a multicentre, open-label, phase 1b study. Lancet Oncol. 2020;21:373–386. doi: 10.1016/S1470-2045(19)30785-5. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

