Interleukin-33-Enhanced CXCR4 Signaling Circuit Mediated by Carcinoma-Associated Fibroblasts Promotes Invasiveness of Head and Neck Cancer
- PMID: 34298657
- PMCID: PMC8306357
- DOI: 10.3390/cancers13143442
Interleukin-33-Enhanced CXCR4 Signaling Circuit Mediated by Carcinoma-Associated Fibroblasts Promotes Invasiveness of Head and Neck Cancer
Abstract
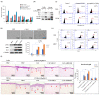
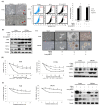
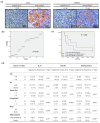
Despite recent advances, treatment for head and neck squamous cell carcinoma (HNSCC) has limited efficacy in preventing tumor progression. We confirmed previously that carcinoma-associated fibroblasts (CAF)-induced interleukin-33 (IL-33) contributed to cancer progression. However, the molecular mechanisms underlying the complex communication network of the tumor microenvironment merited further evaluation. To simulate the IL-33-induced autocrine signaling, stable clones of IL-33-overexpressing HNSCC cells were established. Besides well-established IL-33/ST2 and SDF1/CXCR4 (stromal-derived factor 1/C-X-C motif chemokine receptor 4) signaling, the CAF-induced IL-33 upregulated CXCR4 via cancer cell induction of IL-33 self-production. The IL-33-enhanced-CXCR4 regulatory circuit involves SDF1/CXCR4 signaling activation and modulates tumor behavior. An in vivo study confirmed the functional role of IL-33/CXCR4 in tumor initiation and metastasis. The CXCR4 and/or IL-33 blockade reduced HNSCC cell aggressiveness, with attenuated invasions and metastases. Immunohistochemistry confirmed that IL-33 and CXCR4 expression correlated significantly with disease-free survival and IL-33-CXCR4 co-expression predicted a poor outcome. Besides paracrine signaling, the CAF-induced IL-33 reciprocally enhanced the autocrine cancer-cell self-production of IL-33 and the corresponding CXCR4 upregulation, leading to the activation of SDF1/CXCR4 signaling subsequent to cancer progression. Thus, targeting the IL-33-enhanced-CXCR4 regulatory circuit attenuates tumor aggressiveness and provides a potential therapeutic option for improving the prognosis in HNSCC patients.
Keywords: carcinoma-associated fibroblast; head and neck squamous cell carcinoma; interleukin-33/CXCR4 regulatory circuit; tumor microenvironment; tumor progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

