Targeting Metastatic Colorectal Cancer with Immune Oncological Therapies
- PMID: 34298779
- PMCID: PMC8307556
- DOI: 10.3390/cancers13143566
Targeting Metastatic Colorectal Cancer with Immune Oncological Therapies
Abstract
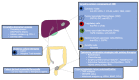
Metastatic colorectal cancer carries poor prognosis, and current therapeutic regimes convey limited improvements in survival and high rates of detrimental side effects in patients that may not stand to benefit. Immunotherapy has revolutionised cancer treatment by restoring antitumoural mechanisms. However, the efficacy in metastatic colorectal cancer, is limited. A literature search was performed using Pubmed (Medline), Web of Knowledge, and Embase. Search terms included combinations of immunotherapy and metastatic colorectal cancer, primarily focusing on clinical trials in humans. Analysis of these studies included status of MMR/MSS, presence of combination strategies, and disease control rate and median overall survival. Evidence shows that immune checkpoint inhibitors, such as anti-PD1 and anti-PD-L1, show efficacy in less than 10% of patients with microsatellite stable, MMR proficient colorectal cancer. In the small subset of patients with microsatellite unstable, MMR deficient cancers, response rates were 40-50%. Combination strategies with immunotherapy are under investigation but have not yet restored antitumoural mechanisms to permit durable disease regression. Immunotherapy provides the potential to offer additional strategies to established chemotherapeutic regimes in metastatic colorectal cancer. Further research needs to establish which adjuncts to immune checkpoint inhibition can unpick resistance, and better predict which patients are likely to respond to individualised therapies to not just improve response rates but to temper unwarranted side effects.
Keywords: anti-PD1; colorectal cancer; immune checkpoint inhibitors; immunotherapy; metastases; microsatellite instability; mismatch repair; targeted therapy; tumour microenvironment; tumour-associated macrophages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Innocenti F., Ou F.S., Qu X., Zemla T.J., Niedzwiecki D., Tam R., Mahajan S., Goldberg R.M., Bertagnolli M.M., Blanke C.D., et al. Mutational Analysis of Patients With Colorectal Cancer in CALGB/SWOG 80405 Identifies New Roles of Microsatellite Instability and Tumor Mutational Burden for Patient Outcome. J. Clin. Oncol. 2019;37:1217–1227. doi: 10.1200/JCO.18.01798. - DOI - PMC - PubMed
-
- Heinemann V., von Weikersthal L.F., Decker T., Kiani A., Vehling-Kaiser U., Al-Batran S.E., Heintges T., Lerchenmuller C., Kahl C., Seipelt G., et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–1075. doi: 10.1016/S1470-2045(14)70330-4. - DOI - PubMed
-
- Schwartzberg L.S., Rivera F., Karthaus M., Fasola G., Canon J.L., Hecht J.R., Yu H., Oliner K.S., Go W.Y. PEAK: A randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J. Clin. Oncol. 2014;32:2240–2247. doi: 10.1200/JCO.2013.53.2473. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

