Lineage Plasticity in Cancer: The Tale of a Skin-Walker
- PMID: 34298815
- PMCID: PMC8306016
- DOI: 10.3390/cancers13143602
Lineage Plasticity in Cancer: The Tale of a Skin-Walker
Abstract
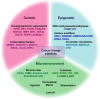
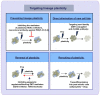
Lineage plasticity, the switching of cells from one lineage to another, has been recognized as a cardinal property essential for embryonic development, tissue repair and homeostasis. However, such a highly regulated process goes awry when cancer cells exploit this inherent ability to their advantage, resulting in tumorigenesis, relapse, metastasis and therapy resistance. In this review, we summarize our current understanding on the role of lineage plasticity in tumor progression and therapeutic resistance in multiple cancers. Lineage plasticity can be triggered by treatment itself and is reported across various solid as well as liquid tumors. Here, we focus on the importance of lineage switching in tumor progression and therapeutic resistance of solid tumors such as the prostate, lung, hepatocellular and colorectal carcinoma and the myeloid and lymphoid lineage switch observed in leukemias. Besides this, we also discuss the role of epithelial-mesenchymal transition (EMT) in facilitating the lineage switch in biphasic cancers such as aggressive carcinosarcomas. We also discuss the mechanisms involved, current therapeutic approaches and challenges that lie ahead in taming the scourge of lineage plasticity in cancer.
Keywords: epithelial-mesenchymal plasticity; lineage plasticity; metastasis; therapy resistance; tumor progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Waddington C.H. The Strategy of the Genes: A Discussion of Some Aspects of Theoretical Biology. Allen & Unwin; London, UK: 1957.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

