Chemotherapy-Induced Myopathy: The Dark Side of the Cachexia Sphere
- PMID: 34298829
- PMCID: PMC8304349
- DOI: 10.3390/cancers13143615
Chemotherapy-Induced Myopathy: The Dark Side of the Cachexia Sphere
Abstract
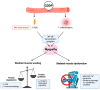
Cancer cachexia is a debilitating multi-factorial wasting syndrome characterised by severe skeletal muscle wasting and dysfunction (i.e., myopathy). In the oncology setting, cachexia arises from synergistic insults from both cancer-host interactions and chemotherapy-related toxicity. The majority of studies have surrounded the cancer-host interaction side of cancer cachexia, often overlooking the capability of chemotherapy to induce cachectic myopathy. Accumulating evidence in experimental models of cachexia suggests that some chemotherapeutic agents rapidly induce cachectic myopathy, although the underlying mechanisms responsible vary between agents. Importantly, we highlight the capacity of specific chemotherapeutic agents to induce cachectic myopathy, as not all chemotherapies have been evaluated for cachexia-inducing properties-alone or in clinically compatible regimens. Furthermore, we discuss the experimental evidence surrounding therapeutic strategies that have been evaluated in chemotherapy-induced cachexia models, with particular focus on exercise interventions and adjuvant therapeutic candidates targeted at the mitochondria.
Keywords: cachexia; chemotherapy; exercise therapy; mitoprotection; muscle wasting; myopathy; pharmaceutical adjuvants; skeletal muscle.
Conflict of interest statement
E.R. is a consultant to Santhera Pharmaceuticals and Epirium Bio. The other authors declare no conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

