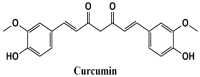
Curcumin Potentiates α7 Nicotinic Acetylcholine Receptors and Alleviates Autistic-Like Social Deficits and Brain Oxidative Stress Status in Mice
- PMID: 34298871
- PMCID: PMC8303708
- DOI: 10.3390/ijms22147251
Curcumin Potentiates α7 Nicotinic Acetylcholine Receptors and Alleviates Autistic-Like Social Deficits and Brain Oxidative Stress Status in Mice
Abstract
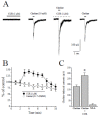
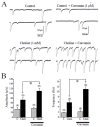
Autistic spectrum disorder (ASD) refers to a group of neurodevelopmental disorders characterized by impaired social interaction and cognitive deficit, restricted repetitive behaviors, altered immune responses, and imbalanced oxidative stress status. In recent years, there has been a growing interest in studying the role of nicotinic acetylcholine receptors (nAChRs), specifically α7-nAChRs, in the CNS. Influence of agonists for α7-nAChRs on the cognitive behavior, learning, and memory formation has been demonstrated in neuro-pathological condition such as ASD and attention-deficit hyperactivity disorder (ADHD). Curcumin (CUR), the active compound of the spice turmeric, has been shown to act as a positive allosteric modulator of α7-nAChRs. Here we hypothesize that CUR, acting through α7-nAChRs, influences the neuropathology of ASD. In patch clamp studies, fast inward currents activated by choline, a selective agonist of α7-nAChRs, were significantly potentiated by CUR. Moreover, choline induced enhancement of spontaneous inhibitory postsynaptic currents was markedly increased in the presence of CUR. Furthermore, CUR (25, 50, and 100 mg/kg, i.p.) ameliorated dose-dependent social deficits without affecting locomotor activity or anxiety-like behaviors of tested male Black and Tan BRachyury (BTBR) mice. In addition, CUR (50 and 100 mg/kg, i.p.) mitigated oxidative stress status by restoring the decreased levels of superoxide dismutase (SOD) and catalase (CAT) in the hippocampus and the cerebellum of treated mice. Collectively, the observed results indicate that CUR potentiates α7-nAChRs in native central nervous system neurons, mitigates disturbed oxidative stress, and alleviates ASD-like features in BTBR mice used as an idiopathic rodent model of ASD, and may represent a promising novel pharmacological strategy for ASD treatment.
Keywords: BTBR mice; autism spectrum disorder; curcumin; nicotinic receptors; oxidative stress; positive allosteric modulator; social features.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Wang L., Almeida L.E.F., Spornick N.A., Kenyon N., Kamimura S., Khaibullina A., Nouraie M., Quezado Z.M.N. Modulation of social deficits and repetitive behaviors in a mouse model of autism: The role of the nicotinic cholinergic system. Psychopharmacology. 2015;232:4303–4316. doi: 10.1007/s00213-015-4058-z. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

