Glucocorticoids, Stress and Delta-9 Tetrahydrocannabinol (THC) during Early Embryonic Development
- PMID: 34298908
- PMCID: PMC8307766
- DOI: 10.3390/ijms22147289
Glucocorticoids, Stress and Delta-9 Tetrahydrocannabinol (THC) during Early Embryonic Development
Abstract
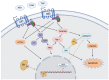
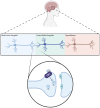
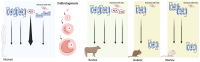
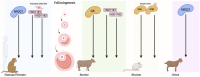
Elevated molecular stress in women is known to have negative impacts on the reproductive development of oocytes and the embryos prior to implantation. In recent years, the prevalence of cannabis use among women of reproductive age has risen due to its ability to relieve psychological stress and nausea, which are mediated by its psychoactive component, ∆-9-tetrahydrocannabinol (THC). Although cannabis is the most popular recreational drug of the 21st century, much is unknown about its influence on molecular stress in reproductive tissues. The current literature has demonstrated that THC causes dose- and time-dependent alterations in glucocorticoid signaling, which have the potential to compromise morphology, development, and quality of oocytes and embryos. However, there are inconsistencies across studies regarding the mechanisms for THC-dependent changes in stress hormones and how either compounds may drive or arrest development. Factors such as variability between animal models, physiologically relevant doses, and undiscovered downstream gene targets of both glucocorticoids and THC could account for such inconsistencies. This review evaluates the results of studies which have investigated the effects of glucocorticoids on reproductive development and how THC may alter stress signaling in relevant tissues.
Keywords: THC; embryos; glucocorticoids; molecular stress; oocytes; pre-implantation development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
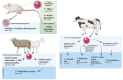
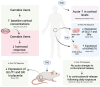
References
-
- National Institute on Drug Abuse. National Survey on Drug Use and Health: Trends in Prevalence of Various Drugs for Ages 12 or Older, Ages 12 to 17, Ages 18 to 25, and Ages 26 or Older; 2015–2017 (in Percent) [(accessed on 16 May 2021)];2018 Available online: https://www.samhsa.gov/data/data-we-collect/nsduh-national-survey-drug-u....
-
- Statistics Canada. Table 13-10-0383-01- Prevalence of Cannabis Use in the Past Three Months, Self-Reported. [(accessed on 20 May 2021)];2021 Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310038301.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

