Chondroprotective Effects of a Histone Deacetylase Inhibitor, Panobinostat, on Pain Behavior and Cartilage Degradation in Anterior Cruciate Ligament Transection-Induced Experimental Osteoarthritic Rats
- PMID: 34298911
- PMCID: PMC8306086
- DOI: 10.3390/ijms22147290
Chondroprotective Effects of a Histone Deacetylase Inhibitor, Panobinostat, on Pain Behavior and Cartilage Degradation in Anterior Cruciate Ligament Transection-Induced Experimental Osteoarthritic Rats
Abstract
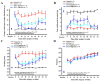
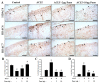
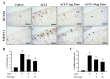
Osteoarthritis (OA) is the most common articular degenerative disease characterized by chronic pain, joint inflammation, and movement limitations, which are significantly influenced by aberrant epigenetic modifications of numerous OA-susceptible genes. Recent studies revealed that both the abnormal activation and differential expression of histone deacetylases (HDACs) might contribute to OA pathogenesis. In this study, we investigated the chondroprotective effects of a marine-derived HDAC inhibitor, panobinostat, on anterior cruciate ligament transection (ACLT)-induced experimental OA rats. The intra-articular administration of 2 or 10 µg of panobinostat (each group, n = 7) per week from the 6th to 17th week attenuates ACLT-induced nociceptive behaviors, including secondary mechanical allodynia and weight-bearing distribution. Histopathological and microcomputed tomography analysis showed that panobinostat significantly prevents cartilage degeneration after ACLT. Moreover, intra-articular panobinostat exerts hypertrophic effects in the chondrocytes of articular cartilage by regulating the protein expressions of HDAC4, HDAC6, HDAC7, runt-domain transcription factor-2, and matrix metalloproteinase-13. The study indicated that HDACs might have different modulations on the chondrocyte phenotype in the early stages of OA development. These results provide new evidence that panobinostat may be a potential therapeutic drug for OA.
Keywords: histone deacetylases; nociception; osteoarthritis; panobinostat.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rubiolo J., Alonso E., Cagide E. Seafood and Freshwater Toxins. CRC Press; Boca Raton, FL, USA: 2014. Marine compounds as a starting point to drugs; pp. 1141–1178.
-
- Quiñoà E., Crews P. Phenolic constituents of Psammaplysilla. Tetrahedron Lett. 1987;28:3229–3232. doi: 10.1016/S0040-4039(00)95478-9. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

