Biocompatibility and Biological Performance Evaluation of Additive-Manufactured Bioabsorbable Iron-Based Porous Suture Anchor in a Rabbit Model
- PMID: 34298988
- PMCID: PMC8307211
- DOI: 10.3390/ijms22147368
Biocompatibility and Biological Performance Evaluation of Additive-Manufactured Bioabsorbable Iron-Based Porous Suture Anchor in a Rabbit Model
Abstract
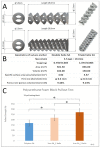
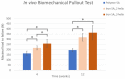
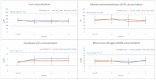
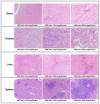
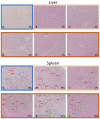
This study evaluated the biocompatibility and biological performance of novel additive-manufactured bioabsorbable iron-based porous suture anchors (iron_SAs). Two types of bioabsorbable iron_SAs, with double- and triple-helical structures (iron_SA_2_helix and iron_SA_3_helix, respectively), were compared with the synthetic polymer-based bioabsorbable suture anchor (polymer_SAs). An in vitro mechanical test, MTT assay, and scanning electron microscope (SEM) analysis were performed. An in vivo animal study was also performed. The three types of suture anchors were randomly implanted in the outer cortex of the lateral femoral condyle. The ultimate in vitro pullout strength of the iron_SA_3_helix group was significantly higher than the iron_SA_2_helix and polymer_SA groups. The MTT assay findings demonstrated no significant cytotoxicity, and the SEM analysis showed cells attachment on implant surface. The ultimate failure load of the iron_SA_3_helix group was significantly higher than that of the polymer_SA group. The micro-CT analysis indicated the iron_SA_3_helix group showed a higher bone volume fraction (BV/TV) after surgery. Moreover, both iron SAs underwent degradation with time. Iron_SAs with triple-helical threads and a porous structure demonstrated better mechanical strength and high biocompatibility after short-term implantation. The combined advantages of the mechanical superiority of the iron metal and the possibility of absorption after implantation make the iron_SA a suitable candidate for further development.
Keywords: additive manufacturing (3D printing); bioabsorbable; iron-based; suture anchor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Park J.B., Bronzino J.D. Biomaterials: Principles and Applications. 1st ed. CRC Press; Boca Raton, FL, USA: 2002.
-
- Khan W., Muntimadugu E., Jaffe M., Domb A.J. Focal Controlled Drug Delivery. Springer; Berlin/Heidelberg, Germany: 2014. Implantable medical devices; pp. 33–59. - DOI
-
- Han H.-S., Loffredo S., Jun I., Edwards J., Kim Y.-C., Seok H.-K., Witte F., Mantovani D., Glyn-Jones S. Current status and outlook on the clinical translation of biodegradable metals. Mater. Today. 2019;23:57–71. doi: 10.1016/j.mattod.2018.05.018. - DOI
-
- Shuai C., Li S., Peng S., Feng P., Lai Y., Gao C. Biodegradable metallic bone implants. Mater. Chem. Front. 2019;3:544–562. doi: 10.1039/C8QM00507A. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

