The MEK5/ERK5 Pathway in Health and Disease
- PMID: 34299213
- PMCID: PMC8303459
- DOI: 10.3390/ijms22147594
The MEK5/ERK5 Pathway in Health and Disease
Abstract
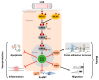
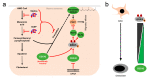
The MEK5/ERK5 mitogen-activated protein kinases (MAPK) cascade is a unique signaling module activated by both mitogens and stress stimuli, including cytokines, fluid shear stress, high osmolarity, and oxidative stress. Physiologically, it is mainly known as a mechanoreceptive pathway in the endothelium, where it transduces the various vasoprotective effects of laminar blood flow. However, it also maintains integrity in other tissues exposed to mechanical stress, including bone, cartilage, and muscle, where it exerts a key function as a survival and differentiation pathway. Beyond its diverse physiological roles, the MEK5/ERK5 pathway has also been implicated in various diseases, including cancer, where it has recently emerged as a major escape route, sustaining tumor cell survival and proliferation under drug stress. In addition, MEK5/ERK5 dysfunction may foster cardiovascular diseases such as atherosclerosis. Here, we highlight the importance of the MEK5/ERK5 pathway in health and disease, focusing on its role as a protective cascade in mechanical stress-exposed healthy tissues and its function as a therapy resistance pathway in cancers. We discuss the perspective of targeting this cascade for cancer treatment and weigh its chances and potential risks when considering its emerging role as a protective stress response pathway.
Keywords: Krüppel-like factor; atherosclerosis; bone; cartilage; endothelium; extracellular-regulated kinase 5; mechanotransduction; mitogen-activated protein kinase; stress signaling; tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

