Magnetic Particle Imaging: Current and Future Applications, Magnetic Nanoparticle Synthesis Methods and Safety Measures
- PMID: 34299271
- PMCID: PMC8306580
- DOI: 10.3390/ijms22147651
Magnetic Particle Imaging: Current and Future Applications, Magnetic Nanoparticle Synthesis Methods and Safety Measures
Abstract
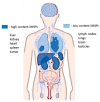
Magnetic nanoparticles (MNPs) have a wide range of applications; an area of particular interest is magnetic particle imaging (MPI). MPI is an imaging modality that utilizes superparamagnetic iron oxide particles (SPIONs) as tracer particles to produce highly sensitive and specific images in a broad range of applications, including cardiovascular, neuroimaging, tumor imaging, magnetic hyperthermia and cellular tracking. While there are hurdles to overcome, including accessibility of products, and an understanding of safety and toxicity profiles, MPI has the potential to revolutionize research and clinical biomedical imaging. This review will explore a brief history of MPI, MNP synthesis methods, current and future applications, and safety concerns associated with this newly emerging imaging modality.
Keywords: magnetic nanoparticles; magnetic particle imaging; nanoparticle safety; superparamagnetic iron oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Williams H. The application of magnetic nanoparticles in the treatment and monitoring of cancer and infectious diseases. Biosci. Horiz. Int. J. Stud. Res. 2017;10:1–10. doi: 10.1093/biohorizons/hzx009. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

