Metabolic Pathways Involved in Formation of Spontaneous and Lipopolysaccharide-Induced Neutrophil Extracellular Traps (NETs) Differ in Obesity and Systemic Inflammation
- PMID: 34299338
- PMCID: PMC8303382
- DOI: 10.3390/ijms22147718
Metabolic Pathways Involved in Formation of Spontaneous and Lipopolysaccharide-Induced Neutrophil Extracellular Traps (NETs) Differ in Obesity and Systemic Inflammation
Abstract
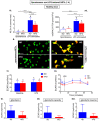
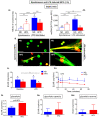
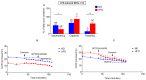
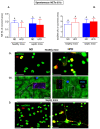
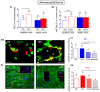
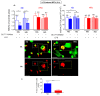
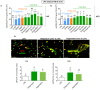
Obesity manifests itself with low-grade chronic inflammation that shapes immune responses during infection. Albeit obese individuals are at risk of higher mortality due to comorbidities, they are better protected from systemic inflammation. Recently, we showed that in the vasculature of obese mice kept on high-fat diet (HFD), neutrophils produce less neutrophil extracellular traps (NETs) than in lean controls (normal diet, ND). NETs are used by neutrophils to counteract severe infection, but they also cause collateral damage. Hardly anything is known about metabolic requirements for their formation, especially in the context of obesity and/or sepsis. Thus, we aimed to study the immunometabolism of NET formation by application of ex vivo neutrophil analyses (Seahorse analyzer, selective inhibitors, confocal imaging) and intravital microscopy. The obtained data show that glycolysis and/or pentose phosphate pathway are involved in NETs release by ND neutrophils in both physiological and inflammatory conditions. In contrast, such cells of septic HFD mice utilize these routes only to spontaneously cast NETs, while after secondary ex vivo activation they exhibit so called "exhausted phenotype", which manifests itself in diminished NET release despite high glycolytic potential and flexibility to oxidize fatty acids. Moreover, impact of ATP synthase inhibition on NET formation is revealed. Overall, the study shows that the neutrophil potential to cast NETs depends on both the metabolic and inflammatory state of the individual.
Keywords: ATP synthase; GLUT1; PPP pathway; fatty acids; glycolysis; immunometabolism; neutrophil extracellular traps; neutrophils; obesity; systemic inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Controlling the Global Obesity Epidemic. [(accessed on 4 May 2021)]; Available online: https://www.who.int/activities/controlling-the-global-obesity-epidemic.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

