Electrochemical Determination of the "Furanic Index" in Honey
- PMID: 34299390
- PMCID: PMC8307740
- DOI: 10.3390/molecules26144115
Electrochemical Determination of the "Furanic Index" in Honey
Abstract
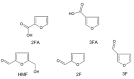
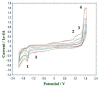
5-(hydroxymethyl)furan-2-carbaldehyde, better known as hydroxymethylfurfural (HMF), is a well-known freshness parameter of honey: although mostly absent in fresh samples, its concentration tends to increase naturally with aging. However, high quantities of HMF are also found in fresh but adulterated samples or honey subjected to thermal or photochemical stresses. In addition, HMF deserves further consideration due to its potential toxic effects on human health. The processes at the origin of HMF formation in honey and in other foods, containing saccharides and proteins-mainly non-enzymatic browning reactions-can also produce other furanic compounds. Among others, 2-furaldehyde (2F) and 2-furoic acid (2FA) are the most abundant in honey, but also their isomers (i.e., 3-furaldehyde, 3F, and 3-furoic acid, 3FA) have been found in it, although in small quantities. A preliminary characterization of HMF, 2F, 2FA, 3F, and 3FA by cyclic voltammetry (CV) led to hypothesizing the possibility of a comprehensive quantitative determination of all these compounds using a simple and accurate square wave voltammetry (SWV) method. Therefore, a new parameter able to provide indications on quality of honey, named "Furanic Index" (FI), was proposed in this contribution, which is based on the simultaneous reduction of all analytes on an Hg electrode to ca. -1.50 V vs. Saturated Calomel Electrode (SCE). The proposed method, validated, and tested on 10 samples of honeys of different botanical origin and age, is fast and accurate, and, in the case of strawberry tree honey (Arbutus unedo), it highlighted the contribution to the FI of the homogentisic acid (HA), i.e., the chemical marker of the floral origin of this honey, which was quantitatively reduced in the working conditions. Excellent agreement between the SWV and Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC) data was observed in all samples considered.
Keywords: HMF; RP-HPLC; cyclic voltammetry; furanic acids; furanic aldehydes; homogentisic acid; honey; square wave voltammetry.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Sanna G., Pilo M.I., Piu P.C., Tapparo A., Seeber R. Determination of heavy metals in honey by anodic stripping voltammetry at microelectrodes. Anal. Chim. Acta. 2000;415:165–173. doi: 10.1016/S0003-2670(00)00864-3. - DOI
-
- Kuster B.F.M. 5-Hydroxymethylfurfural (HMF). A review focusing on its manufacture. Starch Stäerke. 1990;42:314–321. doi: 10.1002/star.19900420808. - DOI
-
- Markowicz D.B., Monaro E., Siguemoto E., Séfora M., Valdez B. Maillard reaction products in processed foods: Pros and cons. In: Valdez B., editor. Food Industrial Processes—Methods and Equipment. 1st ed. InTech; Rijeka, Croatian: 2012. pp. 281–300.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

