Staphylococcus aureus Biofilm: Morphology, Genetics, Pathogenesis and Treatment Strategies
- PMID: 34300053
- PMCID: PMC8304105
- DOI: 10.3390/ijerph18147602
Staphylococcus aureus Biofilm: Morphology, Genetics, Pathogenesis and Treatment Strategies
Abstract
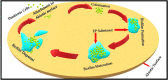
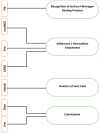
Staphylococcus aureus is a nosocomial bacterium causing different infectious diseases, ranging from skin and soft tissue infections to more serious and life-threatening infections such as septicaemia. S. aureus forms a complex structure of extracellular polymeric biofilm that provides a fully secured and functional environment for the formation of microcolonies, their sustenance and recolonization of sessile cells after its dispersal. Staphylococcus aureus biofilm protects the cells against hostile conditions, i.e., changes in temperature, limitations or deprivation of nutrients and dehydration, and, more importantly, protects the cells against antibacterial drugs. Drugs are increasingly becoming partially or fully inactive against S. aureus as they are either less penetrable or totally impenetrable due to the presence of biofilms surrounding the bacterial cells. Other factors, such as evasion of innate host immune system, genome plasticity and adaptability through gene evolution and exchange of genetic material, also contribute to the ineffectiveness of antibacterial drugs. This increasing tolerance to antibiotics has contributed to the emergence and rise of antimicrobial resistance (AMR), a serious problem that has resulted in increased morbidity and mortality of human and animal populations globally, in addition to causing huge financial losses to the global economy. The purpose of this review is to highlight different aspects of S. aureus biofilm formation and its overall architecture, individual biofilm constituents, clinical implications and role in pathogenesis and drug resistance. The review also discusses different techniques used in the qualitative and quantitative investigation of S. aureus biofilm and various strategies that can be employed to inhibit and eradicate S. aureus biofilm.
Keywords: Staphylococcus aureus; antibiofilm agents; antimicrobial resistance; biofilm formation; gene expression; pathogenesis; quorum sensing.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis or interpretation of the data; in the writing of the manuscript or in the decision to publish the results.
Figures
References
-
- Adams M. Staphylococcus aureus and other pathogenic Gram-positive cocci. In: de Blackburn C.W., McClure P.J., editors. Foodborne Pathogens. Elsevier; Cambridge, UK: 2009. pp. 802–819. Woodhead Publishing Series in Food Science, Technology and Nutrition.
-
- Schaumburg F., Pauly M., Anoh E., Mossoun A., Wiersma L., Schubert G., Flammen A., Alabi A.S., Muyembe-Tamfum J.-J., Grobusch M.P., et al. Staphylococcus aureus complex from animals and humans in three remote African regions. Clin. Microbiol. Infect. 2015;21:345.e1–345.e8. doi: 10.1016/j.cmi.2014.12.001. - DOI - PubMed
-
- Kaplan J.B., Mlynek K.D., Hettiarachchi H., Alamneh Y.A., Biggemann L., Zurawski D.V., Black C.C., Bane C.E., Kim R.K., Granick M.S. Extracellular polymeric substance (EPS)-degrading enzymes reduce staphylococcal surface attachment and biocide resistance on pig skin in vivo. PLoS ONE. 2018;13:e0205526. doi: 10.1371/journal.pone.0205526. - DOI - PMC - PubMed
-
- Nazir R., Zaffar M.R., Amin I. Freshwater Microbiology: Perspectives of Bacterial Dynamics in Lake Ecosystems. Elsevier; Amsterdam, The Netherlands: 2019. Bacterial biofilms: The remarkable heterogeneous biological communities and nitrogen fixing microorganisms in lakes; pp. 307–340.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases