Effectiveness of Mind-Body Intervention for Inflammatory Conditions: Results from a 26-Week Randomized, Non-Blinded, Parallel-Group Trial
- PMID: 34300273
- PMCID: PMC8305779
- DOI: 10.3390/jcm10143107
Effectiveness of Mind-Body Intervention for Inflammatory Conditions: Results from a 26-Week Randomized, Non-Blinded, Parallel-Group Trial
Abstract
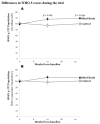
Biopsychosocial intervention has been suggested as a complementary treatment strategy for patients with chronic conditions. We compared the effect of a mind-body intervention (MBI), relative to treatment-as-usual (TAU) on WHO-5 Well-being Index during an intensive period of 12 weeks and follow-up at week 26 among patients with either psoriasis (PsO) or rheumatoid arthritis (RA). The MBI was based on the 'Relaxation Response Resiliency Program' and the 'Open and Calm Program', as well as 'Mindfulness Based Stress Reduction' (MBSR). The trial was randomized, management-as-usual, and controlled. Statistical analyses were based on the intention-to-treat population using repeated measures and mixed effects models (NCT03888261). We screened 39 potential participants, 35 of which (PsO, n = 20; RA, n = 15) met the eligibility criteria and were randomized: 17 in the MBI group and 18 in the TAU group. Attrition from the intervention program was 19%, with 65% of MBI patients and 71% of TAU patients completing the outcome assessments. After 12 weeks, a statistically significant difference in WHO-5 was observed between the groups (p = 0.019). However, according to the protocol, during the entire trial period, the average (least squares mean values) WHO-5 score was higher although not statistically significant in the MBI group (65.3) compared with the TAU group (59.1), corresponding to a between-group difference over 26 weeks of 6.15 (95% CI: -0.26 to 12.56; p = 0.060). All things considered, adding biopsychosocial intervention to clinical practice to patients with conditions, such as PsO and RA, could potentially improve health-related quality of life.
Keywords: biopsychosocial; health-related quality of life; mind–body intervention; psoriasis; rheumatoid arthritis; treatment-as-usual.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form. L.S. has been a paid speaker for AbbVie, Eli Lilly, Novartis, and LEO Pharma, and has been a consultant or has served on Advisory Boards with AbbVie, Janssen Cilag, Novartis, Eli Lilly, LEO Pharma, UCB, Bristol-Myers Squibb, and Sanofi. She has served as an investigator for AbbVie, Sanofi, Janssen Cilag, Boehringer Ingelheim, AstraZeneca, Eli Lilly, Novartis, Regeneron, and LEO Pharma, and has received research and educational grants from Novartis, Sanofi, Bristol-Myers Squibb, Janssen Cilag, and LEO Pharma. No conflicts in relation to this study. C.G.J. is the CEO for the Foundation for Mental Health, which works on a non-profit basis with the Open and Calm-program and MBSR-inspired programs. C.G.J. receives no royalties of any kind related to either program. B.D. has been consultant or has served on Advisory Boards with AbbVie, Novartis, Gilead, Eli Lilly, and Pfizer. No conflicts in relation to this study. The rest of the authors report no conflict of interest.
Figures
References
-
- Naylor C., Parsonage M., McDaid D., Knapp M., Fossey M., Galea A. Long-Term Conditions and Mental Health: The Cost of Co-Morbidities. [(accessed on 8 December 2020)];2012 Available online: http://www.kingsfund.org.uk/publications/mental_health_ltcs.html.
-
- Nast A., Gisondi P., Ormerod A.D., Saiag P., Smith C.H., Spuls P.I., Arenberger P., Bachelez H., Barker J., Dauden E. European s3-guidelines on the systemic treatment of psoriasis vulgaris-update 2015-short version-edf in cooperation with eadv and ipc. J. Eur. Acad. Dermatol. Venereol. 2015;29:2277–2294. doi: 10.1111/jdv.13354. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous