A Cu(II)-MOF Based on a Propargyl Carbamate-Functionalized Isophthalate Ligand as Nitrite Electrochemical Sensor
- PMID: 34300663
- PMCID: PMC8309846
- DOI: 10.3390/s21144922
A Cu(II)-MOF Based on a Propargyl Carbamate-Functionalized Isophthalate Ligand as Nitrite Electrochemical Sensor
Abstract
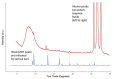
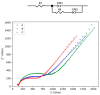
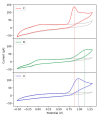
This paper investigates the electrochemical properties of a new Cu(II)-based metal-organic framework (MOF). Noted as Cu-YBDC, it is built upon a linker containing the propargyl carbamate functionality and immobilized on a glassy carbon electrode by drop-casting (GC/Cu-YBDC). Afterward, GC/Cu-YBDC was treated with HAuCl4 and the direct electro-deposition of Au nanoparticles was carried at 0.05 V for 600 s (GC/Au/Cu-YBDC). The performance of both electrodes towards nitrite oxidation was tested and it was found that GC/Au/Cu-YBDC exhibited a better electrocatalytic behavior toward the oxidation of nitrite than GC/Cu-YBDC with enhanced catalytic currents and a reduced nitrite overpotential from 1.20 to 0.90 V. Additionally GC/Au/Cu-YBDC showed a low limit of detection (5.0 μM), an ultrafast response time (<2 s), and a wide linear range of up to 8 mM in neutral pH.
Keywords: electroactive metal-organic frameworks; electrocatalysis; electrochemical sensor; nitrite detection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
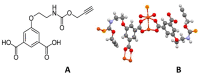




References
-
- Cirujano F.G., Martin N., Wee L.H. Design of hierarchical architectures in metal-oganic frameworks for catalysis and adsorption. Chem. Mater. 2020;32:10268–10295. doi: 10.1021/acs.chemmater.0c02973. - DOI
-
- Shahrokhian S., Ezzati M., Hosseini H. Fabrication of a sensitive and fast response electrochemical glucose sensing platform based on co-based metal-organic frameworks obtained from rapid in situ conversion of electrodeposited cobalt hydroxide intermediates. Talanta. 2020;210:120696. doi: 10.1016/j.talanta.2019.120696. - DOI - PubMed
-
- Zheng W., Liu Y., Yang P., Chen Y., Tao J., Hu J., Zhao P. Carbon nanohorns enhanced electrochemical properties of Cu-based metal organic framework for ultrasensitive serum glucose sensing. J. Electroanal. Chem. 2020;862:114018. doi: 10.1016/j.jelechem.2020.114018. - DOI
-
- Liu B., Wang X., Zhai Y., Zhang Z., Liu H., Li L., Wen H. Facile preparation of well conductive 2D MOF for nonenzymatic detection of hydrogen peroxide: Relationship between electrocatalysis and metal center. J. Electroanal. Chem. 2020;858:113804. doi: 10.1016/j.jelechem.2019.113804. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

