A Review of Plasma Synthesis Methods for Polymer Films and Nanoparticles under Atmospheric Pressure Conditions
- PMID: 34301024
- PMCID: PMC8309454
- DOI: 10.3390/polym13142267
A Review of Plasma Synthesis Methods for Polymer Films and Nanoparticles under Atmospheric Pressure Conditions
Abstract
In this paper, we present an overview of recent approaches in the gas/aerosol-through-plasma (GATP) and liquid plasma methods for synthesizing polymer films and nanoparticles (NPs) using an atmospheric-pressure plasma (APP) technique. We hope to aid students and researchers starting out in the polymerization field by compiling the most commonly utilized simple plasma synthesis methods, so that they can readily select a method that best suits their needs. Although APP methods are widely employed for polymer synthesis, and there are many related papers for specific applications, reviews that provide comprehensive coverage of the variations of APP methods for polymer synthesis are rarely reported. We introduce and compile over 50 recent papers on various APP polymerization methods that allow us to discuss the existing challenges and future direction of GATP and solution plasma methods under ambient air conditions for large-area and mass nanoparticle production.
Keywords: atmospheric-pressure plasma; nanoparticles; plasma polymerization; polymer films; room temperature growth; solution plasma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
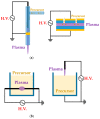
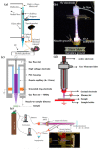
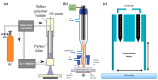
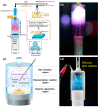







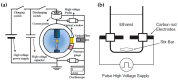



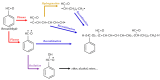


References
-
- The Plasma Chemistry of Polymer Surfaces: Advanced Techniques for Surface Design. 1st ed. Wiley-VCH; Weinheim, Germany: 2021. Plasma polymerization; pp. 337–375.
-
- de Wilde P. Vermischte mittheilungen. Berichte. 1876;7:352.
-
- Berthelot M. Formation de l’acétylène dans les combustions incomplètes. Ann. Chim. Phys. 1866;9:413.
-
- Berthelot M. Action Réciproque des carbures d’hydrogène. synthèse de styrolène, de la napthaline, de l’anthracéne. Ann. Chim. Phys. 1867;12:5–52.
-
- Thenard P., Thenard A. Acétylène liquéfié et solidifié sous l’influence de l’effluve électrique. Comptes Rendus. 1874;78:219.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

