Mechanical Energy Sensing and Harvesting in Micromachined Polymer-Based Piezoelectric Transducers for Fully Implanted Hearing Systems: A Review
- PMID: 34301034
- PMCID: PMC8309449
- DOI: 10.3390/polym13142276
Mechanical Energy Sensing and Harvesting in Micromachined Polymer-Based Piezoelectric Transducers for Fully Implanted Hearing Systems: A Review
Abstract
The paper presents a comprehensive review of mechanical energy harvesters and microphone sensors for totally implanted hearing systems. The studies on hearing mechanisms, hearing losses and hearing solutions are first introduced to bring to light the necessity of creating and integrating the in vivo energy harvester and implantable microphone into a single chip. The in vivo energy harvester can continuously harness energy from the biomechanical motion of the internal organs. The implantable microphone executes mechanoelectrical transduction, and an array of such structures can filter sound frequency directly without an analogue-to-digital converter. The revision of the available transduction mechanisms, device configuration structures and piezoelectric material characteristics reveals the advantage of adopting the polymer-based piezoelectric transducers. A dual function of sensing the sound signal and simultaneously harvesting vibration energy to power up its system can be attained from a single transducer. Advanced process technology incorporates polymers into piezoelectric materials, initiating the invention of a self-powered and flexible transducer that is compatible with the human body, magnetic resonance imaging system (MRI) and the standard complementary metal-oxide-semiconductor (CMOS) processes. The polymer-based piezoelectric is a promising material that satisfies many of the requirements for obtaining high performance implantable microphones and in vivo piezoelectric energy harvesters.
Keywords: MEMS sensor; energy harvester; hearing aids; implantable microphone; piezoelectric-polymers.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



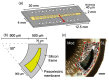






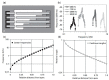



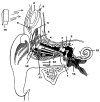

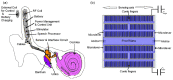





References
-
- Middle Ear Implant. [(accessed on 29 June 2021)]; Available online: https://www.medel.com/hearing-solutions/vibrant-soundbridge.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

