miR-200 deficiency promotes lung cancer metastasis by activating Notch signaling in cancer-associated fibroblasts
- PMID: 34301766
- PMCID: PMC8336896
- DOI: 10.1101/gad.347344.120
miR-200 deficiency promotes lung cancer metastasis by activating Notch signaling in cancer-associated fibroblasts
Abstract
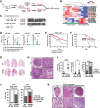
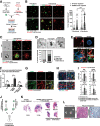
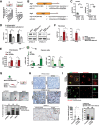
Lung adenocarcinoma, the most prevalent lung cancer subtype, is characterized by its high propensity to metastasize. Despite the importance of metastasis in lung cancer mortality, its underlying cellular and molecular mechanisms remain largely elusive. Here, we identified miR-200 miRNAs as potent suppressors for lung adenocarcinoma metastasis. miR-200 expression is specifically repressed in mouse metastatic lung adenocarcinomas, and miR-200 decrease strongly correlates with poor patient survival. Consistently, deletion of mir-200c/141 in the KrasLSL-G12D/+ ; Trp53flox/flox lung adenocarcinoma mouse model significantly promoted metastasis, generating a desmoplastic tumor stroma highly reminiscent of metastatic human lung cancer. miR-200 deficiency in lung cancer cells promotes the proliferation and activation of adjacent cancer-associated fibroblasts (CAFs), which in turn elevates the metastatic potential of cancer cells. miR-200 regulates the functional interaction between cancer cells and CAFs, at least in part, by targeting Notch ligand Jagged1 and Jagged2 in cancer cells and inducing Notch activation in adjacent CAFs. Hence, the interaction between cancer cells and CAFs constitutes an essential mechanism to promote metastatic potential.
Keywords: Jag1; Jag2; cancer-associated fibroblasts; lung cancer; metastasis; miR-141; miR-200; miR-200c; miRNA; microenvironment.
© 2021 Xue et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
