Therapeutic Targeting of α 7 Nicotinic Acetylcholine Receptors
- PMID: 34301823
- PMCID: PMC8318519
- DOI: 10.1124/pharmrev.120.000097
Therapeutic Targeting of α 7 Nicotinic Acetylcholine Receptors
Abstract
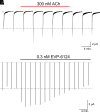
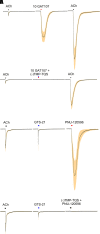
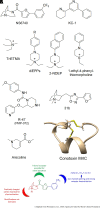
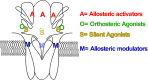
The α7-type nicotinic acetylcholine receptor is one of the most unique and interesting of all the members of the cys-loop superfamily of ligand-gated ion channels. Since it was first identified initially as a binding site for α-bungarotoxin in mammalian brain and later as a functional homomeric receptor with relatively high calcium permeability, it has been pursued as a potential therapeutic target for numerous indications, from Alzheimer disease to asthma. In this review, we discuss the history and state of the art for targeting α7 receptors, beginning with subtype-selective agonists and the basic pharmacophore for the selective activation of α7 receptors. A key feature of α7 receptors is their rapid desensitization by standard "orthosteric" agonist, and we discuss insights into the conformational landscape of α7 receptors that has been gained by the development of ligands binding to allosteric sites. Some of these sites are targeted by positive allosteric modulators that have a wide range of effects on the activation profile of the receptors. Other sites are targeted by direct allosteric agonist or antagonists. We include a perspective on the potential importance of α7 receptors for metabotropic as well as ionotropic signaling. We outline the challenges that exist for future development of drugs to target this important receptor and approaches that may be considered to address those challenges. SIGNIFICANCE STATEMENT: The α7-type nicotinic acetylcholine receptor (nAChR) is acknowledged as a potentially important therapeutic target with functional properties associated with both ionotropic and metabotropic signaling. The functional properties of α7 nAChR can be regulated in diverse ways with the variety of orthosteric and allosteric ligands described in this review.
Copyright © 2021 by The American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
No author has an actual or perceived conflict of interest with the contents of this article.
Figures










References
-
- Acker BAJacobsen EJRogers BNWishka DGReitz SCPiotrowski DWMyers JKWolfe MLGroppi VEThornburgh BA, et al. (2008) Discovery of N-[(3R,5R)-1-azabicyclo[3.2.1]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide as an agonist of the alpha7 nicotinic acetylcholine receptor: in vitro and in vivo activity. Bioorg Med Chem Lett 18:3611–3615. - PubMed
-
- Adams DJ, Nutter TJ (1992) Calcium permeability and modulation of nicotinic acetylcholine receptor-channels in rat parasympathetic neurons. J Physiol Paris 86:67–76. - PubMed
-
- Aiyar VN, Benn MH, Hanna T, Jacyno J, Roth SH, Wilkens JL (1979) The principal toxin of Delphinium brownii Rydb., and its mode of action. Experientia 35:1367–1368. - PubMed
-
- Alkondon M, Albuquerque EX (1995) Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. III. Agonist actions of the novel alkaloid epibatidine and analysis of type II current. J Pharmacol Exp Ther 274:771–782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

