Genomic evolution of antimicrobial resistance in Escherichia coli
- PMID: 34301966
- PMCID: PMC8302606
- DOI: 10.1038/s41598-021-93970-7
Genomic evolution of antimicrobial resistance in Escherichia coli
Abstract
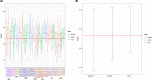
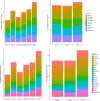
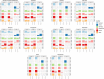
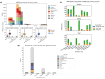
The emergence of antimicrobial resistance (AMR) is one of the biggest health threats globally. In addition, the use of antimicrobial drugs in humans and livestock is considered an important driver of antimicrobial resistance. The commensal microbiota, and especially the intestinal microbiota, has been shown to have an important role in the emergence of AMR. Mobile genetic elements (MGEs) also play a central role in facilitating the acquisition and spread of AMR genes. We isolated Escherichia coli (n = 627) from fecal samples in respectively 25 poultry, 28 swine, and 15 veal calf herds from 6 European countries to investigate the phylogeny of E. coli at country, animal host and farm levels. Furthermore, we examine the evolution of AMR in E. coli genomes including an association with virulence genes, plasmids and MGEs. We compared the abundance metrics retrieved from metagenomic sequencing and whole genome sequenced of E. coli isolates from the same fecal samples and farms. The E. coli isolates in this study indicated no clonality or clustering based on country of origin and genetic markers; AMR, and MGEs. Nonetheless, mobile genetic elements play a role in the acquisition of AMR and virulence genes. Additionally, an abundance of AMR was agreeable between metagenomic and whole genome sequencing analysis for several AMR classes in poultry fecal samples suggesting that metagenomics could be used as an indicator for surveillance of AMR in E. coli isolates and vice versa.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

