BACE inhibitor treatment of mice induces hyperactivity in a Seizure-related gene 6 family dependent manner without altering learning and memory
- PMID: 34302009
- PMCID: PMC8302682
- DOI: 10.1038/s41598-021-94369-0
BACE inhibitor treatment of mice induces hyperactivity in a Seizure-related gene 6 family dependent manner without altering learning and memory
Abstract
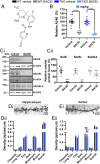
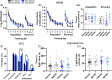
BACE inhibitors, which decrease BACE1 (β-secretase 1) cleavage of the amyloid precursor protein, are a potential treatment for Alzheimer's disease. Clinical trials using BACE inhibitors have reported a lack of positive effect on patient symptoms and, in some cases, have led to increased adverse events, cognitive worsening and hippocampal atrophy. A potential drawback of this strategy is the effect of BACE inhibition on other BACE1 substrates such as Seizure-related gene 6 (Sez6) family proteins which are known to have a role in neuronal function. Mice were treated with an in-diet BACE inhibitor for 4-8 weeks to achieve a clinically-relevant level of amyloid-β40 reduction in the brain. Mice underwent behavioural testing and postmortem analysis of dendritic spine number and morphology with Golgi-Cox staining. Sez6 family triple knockout mice were tested alongside wild-type mice to identify whether any effects of the treatment were due to altered cleavage of Sez6 family proteins. Wild-type mice treated with BACE inhibitor displayed hyperactivity on the elevated open field, as indicated by greater distance travelled, but this effect was not observed in treated Sez6 triple knockout mice. BACE inhibitor treatment did not lead to significant changes in spatial or fear learning, reference memory, cognitive flexibility or anxiety in mice as assessed by the Morris water maze, context fear conditioning, or light-dark box tests. Chronic BACE inhibitor treatment reduced the density of mushroom-type spines in the somatosensory cortex, regardless of genotype, but did not affect steady-state dendritic spine density or morphology in the CA1 region of the hippocampus. Chronic BACE inhibition for 1-2 months in mice led to increased locomotor output but did not alter memory or cognitive flexibility. While the mechanism underlying the treatment-induced hyperactivity is unknown, the absence of this response in Sez6 triple knockout mice indicates that blocking ectodomain shedding of Sez6 family proteins is a contributing factor. In contrast, the decrease in mature spine density in cortical neurons was not attributable to lack of shed Sez6 family protein ectodomains. Therefore, other BACE1 substrates are implicated in this effect and, potentially, in the cognitive decline in longer-term chronically treated patients.
© 2021. The Author(s).
Conflict of interest statement
Harrie Gijsen and Brian Hrupka are employees of Janssen Pharmaceutica NV and Harrie Gijsen holds stock of Johnson & Johnson. All other authors declare they have no competing interests.
Figures

Similar articles
-
Beta-Site Amyloid Precursor Protein Cleaving Enzyme 1 Inhibition Impairs Synaptic Plasticity via Seizure Protein 6.Biol Psychiatry. 2018 Mar 1;83(5):428-437. doi: 10.1016/j.biopsych.2016.12.023. Epub 2016 Dec 26. Biol Psychiatry. 2018. PMID: 28129943
-
New Highly Selective BACE1 Inhibitors and Their Effects on Dendritic Spine Density In Vivo.Int J Mol Sci. 2023 Jul 31;24(15):12283. doi: 10.3390/ijms241512283. Int J Mol Sci. 2023. PMID: 37569661 Free PMC article.
-
Seizure protein 6 and its homolog seizure 6-like protein are physiological substrates of BACE1 in neurons.Mol Neurodegener. 2016 Oct 5;11(1):67. doi: 10.1186/s13024-016-0134-z. Mol Neurodegener. 2016. PMID: 27716410 Free PMC article.
-
Functions of the Alzheimer's Disease Protease BACE1 at the Synapse in the Central Nervous System.J Mol Neurosci. 2016 Nov;60(3):305-315. doi: 10.1007/s12031-016-0800-1. Epub 2016 Jul 25. J Mol Neurosci. 2016. PMID: 27456313 Free PMC article. Review.
-
Consequences of Pharmacological BACE Inhibition on Synaptic Structure and Function.Biol Psychiatry. 2018 Oct 1;84(7):478-487. doi: 10.1016/j.biopsych.2018.04.022. Epub 2018 Jun 23. Biol Psychiatry. 2018. PMID: 29945719 Review.
Cited by
-
Associations of CSF BACE1 with amyloid pathology, neurodegeneration, and cognition in Alzheimer's disease.Acta Neuropathol. 2024 Jun 10;147(1):97. doi: 10.1007/s00401-024-02750-w. Acta Neuropathol. 2024. PMID: 38856925
-
Targeted delivery of BACE1 siRNA for synergistic treatment of Alzheimer's disease.Transl Neurodegener. 2025 Aug 14;14(1):41. doi: 10.1186/s40035-025-00503-7. Transl Neurodegener. 2025. PMID: 40814010 Free PMC article.
-
Epilepsy in Neurodegenerative Diseases: Related Drugs and Molecular Pathways.Pharmaceuticals (Basel). 2021 Oct 18;14(10):1057. doi: 10.3390/ph14101057. Pharmaceuticals (Basel). 2021. PMID: 34681281 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

