The role of IL-1 in adipose browning and muscle wasting in CKD-associated cachexia
- PMID: 34302016
- PMCID: PMC8302616
- DOI: 10.1038/s41598-021-94565-y
The role of IL-1 in adipose browning and muscle wasting in CKD-associated cachexia
Abstract
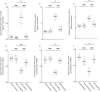
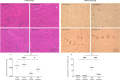
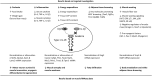
Cytokines such as IL-6, TNF-α and IL-1β trigger inflammatory cascades which may play a role in the pathogenesis of chronic kidney disease (CKD)-associated cachexia. CKD was induced by 5/6 nephrectomy in mice. We studied energy homeostasis in Il1β-/-/CKD, Il6-/-/CKD and Tnfα-/-/CKD mice and compared with wild type (WT)/CKD controls. Parameters of cachexia phenotype were completely normalized in Il1β-/-/CKD mice but were only partially rescued in Il6-/-/CKD and Tnfα-/-/CKD mice. We tested the effects of anakinra, an IL-1 receptor antagonist, on CKD-associated cachexia. WT/CKD mice were treated with anakinra (2.5 mg/kg/day, IP) or saline for 6 weeks and compared with WT/Sham controls. Anakinra normalized food intake and weight gain, fat and lean mass content, metabolic rate and muscle function, and also attenuated molecular perturbations of energy homeostasis in adipose tissue and muscle in WT/CKD mice. Anakinra decreased serum and muscle expression of IL-6, TNF-α and IL-1β in WT/CKD mice. Anakinra attenuated browning of white adipose tissue in WT/CKD mice. Moreover, anakinra normalized gastrocnemius weight and fiber size as well as attenuated muscle fat infiltration in WT/CKD mice. This was accompanied by correcting the increased muscle wasting signaling pathways while promoting the decreased myogenesis process in gastrocnemius of WT/CKD mice. We performed qPCR analysis for the top 20 differentially expressed muscle genes previously identified via RNAseq analysis in WT/CKD mice versus controls. Importantly, 17 differentially expressed muscle genes were attenuated in anakinra treated WT/CKD mice. In conclusion, IL-1 receptor antagonism may represent a novel targeted treatment for adipose tissue browning and muscle wasting in CKD.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that there is no competing conflict of interest, either financial or non-financial as listed in the journal website, that could be perceived as prejudicing the impartiality of the research reported.
Figures






Similar articles
-
Targeting interleukin-1 for reversing fat browning and muscle wasting in infantile nephropathic cystinosis.J Cachexia Sarcopenia Muscle. 2021 Oct;12(5):1296-1311. doi: 10.1002/jcsm.12744. Epub 2021 Jun 30. J Cachexia Sarcopenia Muscle. 2021. PMID: 34196133 Free PMC article.
-
A Leptin Receptor Antagonist Attenuates Adipose Tissue Browning and Muscle Wasting in Infantile Nephropathic Cystinosis-Associated Cachexia.Cells. 2021 Jul 31;10(8):1954. doi: 10.3390/cells10081954. Cells. 2021. PMID: 34440723 Free PMC article.
-
Vitamin D repletion ameliorates adipose tissue browning and muscle wasting in infantile nephropathic cystinosis-associated cachexia.J Cachexia Sarcopenia Muscle. 2020 Feb;11(1):120-134. doi: 10.1002/jcsm.12497. Epub 2019 Nov 13. J Cachexia Sarcopenia Muscle. 2020. PMID: 31721480 Free PMC article.
-
Parathyroid hormone stimulates adipose tissue browning: a pathway to muscle wasting.Curr Opin Clin Nutr Metab Care. 2017 May;20(3):153-157. doi: 10.1097/MCO.0000000000000357. Curr Opin Clin Nutr Metab Care. 2017. PMID: 28257332 Free PMC article. Review.
-
Adipose tissue dysfunction in cancer cachexia.J Cell Physiol. 2018 Jan;234(1):13-22. doi: 10.1002/jcp.26811. Epub 2018 Aug 4. J Cell Physiol. 2018. PMID: 30078199 Review.
Cited by
-
Developing an Evidence and Theory Based Multimodal Integrative Intervention for the Management of Renal Cachexia: A Theory of Change.Healthcare (Basel). 2022 Nov 22;10(12):2344. doi: 10.3390/healthcare10122344. Healthcare (Basel). 2022. PMID: 36553868 Free PMC article.
-
Fibroblast Growth Factor 23 and Muscle Wasting: A Metabolic Point of View.Kidney Int Rep. 2023 May 3;8(7):1301-1314. doi: 10.1016/j.ekir.2023.04.027. eCollection 2023 Jul. Kidney Int Rep. 2023. PMID: 37441473 Free PMC article. Review.
-
Combating chronic kidney disease-associated cachexia: A literature review of recent therapeutic approaches.BMC Nephrol. 2025 Mar 11;26(1):133. doi: 10.1186/s12882-025-04057-8. BMC Nephrol. 2025. PMID: 40069669 Free PMC article. Review.
-
Whole transcriptome expression profiles in kidney samples from rats with hyperuricaemic nephropathy.PLoS One. 2022 Dec 19;17(12):e0276591. doi: 10.1371/journal.pone.0276591. eCollection 2022. PLoS One. 2022. PMID: 36534664 Free PMC article.
-
Nutrition-Focused Physical Examination for Detecting Protein Energy Wasting in Children with Chronic Kidney Disease.Indian J Nephrol. 2023 Jul-Aug;33(4):264-269. doi: 10.4103/ijn.ijn_145_22. Epub 2023 Mar 7. Indian J Nephrol. 2023. PMID: 37781562 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

