Development of thymic tumor in [LSL:KrasG12D; Pdx1-CRE] mice, an adverse effect associated with accelerated pancreatic carcinogenesis
- PMID: 34302028
- PMCID: PMC8302691
- DOI: 10.1038/s41598-021-94566-x
Development of thymic tumor in [LSL:KrasG12D; Pdx1-CRE] mice, an adverse effect associated with accelerated pancreatic carcinogenesis
Abstract
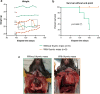
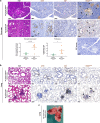
Pancreatic Ductal AdenoCarcinoma (PDAC) represents about 90% of pancreatic cancers. It is one of the most aggressive cancer, with a 5-year survival rate below 10% due to late diagnosis and poor therapeutic efficiency. This bad prognosis thus encourages intense research in order to better understand PDAC pathogenesis and molecular basis leading to the development of innovative therapeutic strategies. This research frequently involves the KC (LSL:KrasG12D;Pdx1-CRE) genetically engineered mouse model, which leads to pancreatic cancer predisposition. However, as frequently encountered in animal models, the KC mouse model also exhibits biases. Herein, we report a new adverse effect of KrasG12D mutation in KC mouse model. In our hands, 10% of KC mice developed clinical signs reaching pre-defined end-points between 100- and 150-days post-parturition, and associated with large thymic mass development. Histological and genetic analyses of this massive thymus enabled us (1) to characterize it as a highly proliferative thymic lymphoma and (2) to detect the unexpected recombination of the Lox-STOP-Lox cassette upstream KrasG12D allele and subsequent KRASG12D protein expression in all cells composing thymic masses. Finally, we highlighted that development of such thymic tumor was associated with accelerated pancreatic carcinogenesis, immune compartment disorganization, and in some cases, lung malignancies.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Eibl, A. S. and G. Pancreatic Ductal Adenocarcinoma. Pancreapedia: The Exocrine Pancreas Knowledge Base (2015). 10.3998/panc.2015.14.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

