Catch me if you can: how AML and its niche escape immunotherapy
- PMID: 34302116
- PMCID: PMC8727297
- DOI: 10.1038/s41375-021-01350-x
Catch me if you can: how AML and its niche escape immunotherapy
Abstract
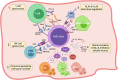
In spite of the remarkable progress in basic and preclinical studies of acute myeloid leukemia (AML), the five-year survival rate of AML patients remains poor, highlighting the urgent need for novel and synergistic therapies. Over the past decade, increased attention has been focused on identifying suitable immunotherapeutic strategies for AML, and in particular on targeting leukemic cells and their progenitors. However, recent studies have also underlined the important contribution of the leukemic microenvironment in facilitating tumor escape mechanisms leading to disease recurrence. Here, we describe the immunological features of the AML niche, with particular attention to the crosstalk between the AML blasts and the cellular components of the altered tumor microenvironment (TME) and the mechanisms of immune escape that hamper the therapeutic effects of the most advanced treatments. Considering the AML complexity, immunotherapy approaches may benefit from a rational combination of complementary strategies aimed at preventing escape mechanisms without increasing toxicity.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

