Machinery, regulation and pathophysiological implications of autophagosome maturation
- PMID: 34302147
- PMCID: PMC8300085
- DOI: 10.1038/s41580-021-00392-4
Machinery, regulation and pathophysiological implications of autophagosome maturation
Abstract
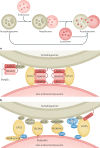
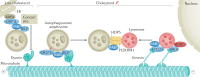
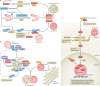
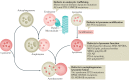
Autophagy is a versatile degradation system for maintaining cellular homeostasis whereby cytosolic materials are sequestered in a double-membrane autophagosome and subsequently delivered to lysosomes, where they are broken down. In multicellular organisms, newly formed autophagosomes undergo a process called 'maturation', in which they fuse with vesicles originating from endolysosomal compartments, including early/late endosomes and lysosomes, to form amphisomes, which eventually become degradative autolysosomes. This fusion process requires the concerted actions of multiple regulators of membrane dynamics, including SNAREs, tethering proteins and RAB GTPases, and also transport of autophagosomes and late endosomes/lysosomes towards each other. Multiple mechanisms modulate autophagosome maturation, including post-translational modification of key components, spatial distribution of phosphoinositide lipid species on membranes, RAB protein dynamics, and biogenesis and function of lysosomes. Nutrient status and various stresses integrate into the autophagosome maturation machinery to coordinate the progression of autophagic flux. Impaired autophagosome maturation is linked to the pathogenesis of various human diseases, including neurodegenerative disorders, cancer and myopathies. Furthermore, invading pathogens exploit various strategies to block autophagosome maturation, thus evading destruction and even subverting autophagic vacuoles (autophagosomes, amphisomes and autolysosomes) for survival, growth and/or release. Here, we discuss the recent progress in our understanding of the machinery and regulation of autophagosome maturation, the relevance of these mechanisms to human pathophysiology and how they are harnessed by pathogens for their benefit. We also provide perspectives on targeting autophagosome maturation therapeutically.
© 2021. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

