Systemic exposure following intravitreal administration of therapeutic agents: an integrated pharmacokinetic approach. 2. THR-687
- PMID: 34302261
- PMCID: PMC8604881
- DOI: 10.1007/s10928-021-09774-9
Systemic exposure following intravitreal administration of therapeutic agents: an integrated pharmacokinetic approach. 2. THR-687
Abstract
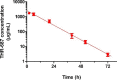
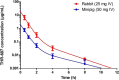
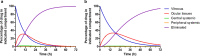
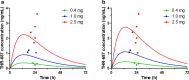
Intravitreal (IVT) injection remains the preferred administration route of pharmacological agents intended for the treatment of back of the eye diseases such as diabetic macular edema (DME) and neovascular age-related macular degeneration (nvAMD). The procedure enables drugs to be delivered locally at high concentrations whilst limiting whole body exposure and associated risk of systemic adverse events. Nevertheless, intravitreally-delivered drugs do enter the general circulation and achieving an accurate understanding of systemic exposure is pivotal for the evaluation and development of drugs administered in the eye. We report here the full pharmacokinetic properties of THR-687, a pan RGD integrin antagonist currently in clinical development for the treatment of DME, in both rabbit and minipig. Pharmacokinetic characterization included description of vitreal elimination, of systemic pharmacokinetics, and of systemic exposure following IVT administration. For the latter, we present a novel pharmacokinetic model that assumes clear partition between the vitreous humor compartment itself where the drug is administered and the central systemic compartment. We also propose an analytical solution to the system of differential equations that represent the pharmacokinetic model, thereby allowing data analysis with standard nonlinear regression analysis. The model accurately describes circulating levels of THR-687 following IVT administration in relevant animal models, and we suggest that this approach is relevant to a range of drugs and analysis of subsequent systemic exposure.
Keywords: Integrated pharmacokinetics; Intravitreal administration; Systemic exposure.
© 2021. The Author(s).
Conflict of interest statement
Jean-Marc Wagner has no financial or non-financial interests to disclose. Marc Vanhove, Bernard Noppen, Bart Jonckx, and Elke Vermassen are employees of Oxurion N.V. Alan Stitt is consultant to Oxurion N.V.
Figures


Similar articles
-
Systemic exposure following intravitreal administration of therapeutic agents: an integrated pharmacokinetic approach. 1. THR-149.J Pharmacokinet Pharmacodyn. 2021 Dec;48(6):825-836. doi: 10.1007/s10928-021-09773-w. Epub 2021 Jul 23. J Pharmacokinet Pharmacodyn. 2021. PMID: 34302260 Free PMC article.
-
A preliminary evaluation of dexamethasone palmitate emulsion: a novel intravitreal sustained delivery of corticosteroid for treatment of macular edema.J Ocul Pharmacol Ther. 2013 Mar;29(2):258-69. doi: 10.1089/jop.2012.0044. Epub 2013 Jan 18. J Ocul Pharmacol Ther. 2013. PMID: 23331052
-
Pharmacokinetics of ranibizumab after intravitreal administration in patients with retinal vein occlusion or diabetic macular edema.Ophthalmology. 2014 Nov;121(11):2237-46. doi: 10.1016/j.ophtha.2014.05.012. Epub 2014 Jul 4. Ophthalmology. 2014. PMID: 25001159 Clinical Trial.
-
Comprehensive Review of Ocular and Systemic Safety Events with Intravitreal Aflibercept Injection in Randomized Controlled Trials.Ophthalmology. 2016 Jul;123(7):1511-20. doi: 10.1016/j.ophtha.2016.02.046. Epub 2016 Apr 12. Ophthalmology. 2016. PMID: 27084563 Review.
-
Update of intravitreal steroids for the treatment of diabetic macular edema.Ophthalmic Res. 2014;52(2):89-96. doi: 10.1159/000362764. Epub 2014 Aug 29. Ophthalmic Res. 2014. PMID: 25195600 Review.
Cited by
-
Phase 1 Dose-Escalation Study of Plasma Kallikrein Inhibitor THR-149 for the Treatment of Diabetic Macular Edema.Transl Vis Sci Technol. 2021 Dec 1;10(14):28. doi: 10.1167/tvst.10.14.28. Transl Vis Sci Technol. 2021. PMID: 34940810 Free PMC article. Clinical Trial.
References
-
- Wang W, Wang F, Qin W, Liu H, Lu B, Chung C, Zhu J, Gu Q, Shi W, Wen C, Wu F, Zhang K, Sun X. Joint antiangiogenic effect of ATN-161 and anti-VEGF antibody in a rat model of early wet age-related macular degeneration. Mol Pharm. 2016;13:2881–2890. doi: 10.1021/acs.molpharmaceut.6b00056. - DOI - PubMed
-
- Varela-Fernández R, Díaz-Tomé V, Luaces-Rodríguez A, Conde-Penedo A, García-Otero X, Luzardo-Álvarez A, Fernández-Ferreiro A, Otero-Espinar FJ. Drug delivery to the posterior segment of the eye: biopharmaceutic and pharmacokinetic considerations. Pharmaceutics. 2020;12:269. doi: 10.3390/pharmaceutics12030269. - DOI - PMC - PubMed
-
- del Amo EM, Rimpelä A-K, Heikkinen E, Kari OK, Ramsay E, Lajunen T, Schmitt M, Pelkonen L, Bhattacharya M, Richardson D, Subrizi A, Turunen T, Reinisalo M, Itkonen J, Toropainen E, Casteleijn M, Kidron H, Antopolsky M, Vellonen K-S, Ruponen M, Urtti A. Pharmacokinetic aspects of retinal drug delivery. Prog Retin Eye Res. 2017;57:134–185. doi: 10.1016/j.preteyeres.2016.12.001. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

