Current hurdles to the translation of nanomedicines from bench to the clinic
- PMID: 34302274
- PMCID: PMC8300981
- DOI: 10.1007/s13346-021-01024-2
Current hurdles to the translation of nanomedicines from bench to the clinic
Abstract
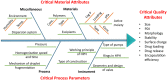
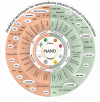
The field of nanomedicine has significantly influenced research areas such as drug delivery, diagnostics, theranostics, and regenerative medicine; however, the further development of this field will face significant challenges at the regulatory level if related guidance remains unclear and unconsolidated. This review describes those features and pathways crucial to the clinical translation of nanomedicine and highlights considerations for early-stage product development. These include identifying those critical quality attributes of the drug product essential for activity and safety, appropriate analytical methods (physical, chemical, biological) for characterization, important process parameters, and adequate pre-clinical models. Additional concerns include the evaluation of batch-to-batch consistency and considerations regarding scaling up that will ensure a successful reproducible manufacturing process. Furthermore, we advise close collaboration with regulatory agencies from the early stages of development to assure an aligned position to accelerate the development of future nanomedicines.
Keywords: Characterization; Manufacturing; Nanomedicine translation; Regulatory framework; Scale-up.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Ashton S, Song YH, Nolan J, Cadogan E, Murray J, Odedra R, et al. Aurora kinase inhibitor nanoparticles target tumors with favorable therapeutic index in vivo. Sci Transl Med. 2016;8:325ra17. 10.1126/scitranslmed.aad2355 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

