Pharmacokinetics of Ceftazidime in Children and Adolescents with Obesity
- PMID: 34302290
- PMCID: PMC9706343
- DOI: 10.1007/s40272-021-00460-4
Pharmacokinetics of Ceftazidime in Children and Adolescents with Obesity
Abstract
Purpose: The aim of this study was to evaluate ceftazidime pharmacokinetics (PK) in a cohort that includes a predominate number of children and adolescents with obesity and assess the efficacy of competing dosing strategies.
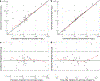
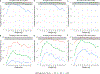
Methods: A population PK model was developed using opportunistically collected plasma samples. For each dosing strategy, model-based probability of target attainment (PTA) estimates were computed for study participants using empirical Bayes estimates. In addition, the effects of body size and renal function on PTA were evaluated using stochastic model simulations with virtually generated subjects.
Results: Twenty-nine participants, 24 of whom were obese, contributed data towards the analysis. The median (range) age, body weight, and body mass index of participants were 12.2 years (2.3-20.6), 59.2 kg (8.4-121), and 25.2 kg/m2 (13.8-42.9), respectively. Administration of 50 mg/kg intravenously (IV) every 8 hours (q8h; max 6 g/day) or 40 mg/kg IV q6h (max 6 g/day) resulted in PTA values of ≥ 90% (minimum inhibitory concentration 8 mg/L) for the subset of obese participants with estimated glomerular filtration rates (GFR) ≥ ~ 80 mL/min/1.73 m2. However, for both regimens, stochastic model simulations denoted lower PTA values (< 90%) with increasing body weight for virtual subjects with GFR ≥ 120 mL/min/1.73 m2. Alternatively, permitting for a maximum daily dose of 8 g/day using a 40 mg/kg IV q6h regimen provided PTA values that were near or above target (90%) for virtual subjects between 10 to 120 kg with GFR ≥ 80 mL/min/1.73 m2.
Conclusion: Our analysis suggests administration of 40 mg/kg IV q6h (max 8 g/day) maximizes PTA in children and adolescents with obesity and GFR ≥ 80 mL/min/1.73 m2.
Trial registration: Clinicaltrials.gov Identifier: NCT01431326.
© 2021. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
Conflicts of Interest:
A.R.M. receives support from the Thrasher Research Fund’s Early Career Award.
C.P.H. receives salary support for research from National Institute for Child Health and Human Development (NICHD) (R13HD102136), the National Heart Lung and Blood Institute (NHLBI) (R61/R33HL147833), the US Food and Drug Administration (R01-FD006099, PI Laughon; and U18-FD006298, PI: Benjamin), the U.S. government for his work in pediatric clinical pharmacology (Government Contract HHSN275201800003I, PI: Benjamin under the Best Pharmaceuticals for Children Act), the non-profit Burroughs Wellcome Fund, and other sponsors for drug development in adults and children (
D.K.B. Jr. receives support from the National Institutes of Health (National Institute of Child Health and Human Development (HHSN275201000003I), the National Center for Advancing Translational Sciences (1U24TR001608), and Food and Drug Administration (1U18FD006298); he also receives research support from industry for neonatal and pediatric drug development.
J.A. receives salary support from the FRQS (Fonds de Recherche Santé Québec), and does consulting for Astellas Pharma Inc.
J.E.S. receives salary support for research the National Institute for Health (NIH) (2 UG1 OD024954 and 1UG1HD090904–01), Environmental Health Sciences Core Centers (EHSCC). NIH 1P30ES030283–01A1, and her work in pediatric clinical pharmacology (subcontract with Duke University under Government Contract HHSN275201800003I), and other sponsors/ industry for drug development in infants and children.
K.O.Z. receives salary support from the National Institutes of Health (National Institute of Child Health and Human Development (K23 HD091398, HHSN275201000003I), the US Food and Drug Administration (UG3/UH3 FD 006797), the Duke Clinical and Translational Science Award (KL2TR001115–03), and industry for neonatal and pediatric drug development (
All other authors do not have relevant conflicts of interest.
Figures
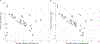

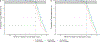
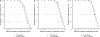
References
-
- Alobaid AS, Hites M, Lipman J, Taccone FS, Roberts JA. Effect of obesity on the pharmacokinetics of antimicrobials in critically ill patients: A structured review. Int J Antimicrob Agents. 2016. Apr;47(4):259–68. - PubMed
-
- Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71–87. - PubMed
-
- Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, et al. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it’s not just for mice anymore. Clin Infect Dis. 2007. Jan 1;44(1):79–86. - PubMed
-
- Barger A, Fuhst C, Wiedemann B. Pharmacological indices in antibiotic therapy. J Antimicrob Chemother. 2003. Dec;52(6):893–8. - PubMed
MeSH terms
Substances
Associated data
Grants and funding
- HHSN267200700051C/HD/NICHD NIH HHS/United States
- P30 ES030283/ES/NIEHS NIH HHS/United States
- UG3 FD006797/FD/FDA HHS/United States
- HHSN275201000003C/HD/NICHD NIH HHS/United States
- K23 HD091398/HD/NICHD NIH HHS/United States
- R01 FD006099/FD/FDA HHS/United States
- KL2 TR001115/TR/NCATS NIH HHS/United States
- HHSN275201000003I/HD/NICHD NIH HHS/United States
- R13 HD102136/HD/NICHD NIH HHS/United States
- R01 HD076676/HD/NICHD NIH HHS/United States
- U24 TR001608/TR/NCATS NIH HHS/United States
- HHSN275201800003I/HD/NICHD NIH HHS/United States
- UG1 HD090904/HD/NICHD NIH HHS/United States
- UG1 OD024954/OD/NIH HHS/United States
- R33 HL147833/HL/NHLBI NIH HHS/United States
- U18 FD006298/FD/FDA HHS/United States
- UH3 FD006797/FD/FDA HHS/United States
LinkOut - more resources
Full Text Sources
Medical

